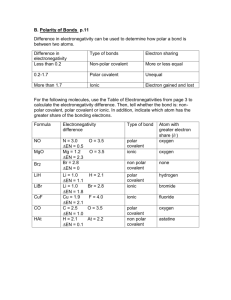
Ch9
advertisement
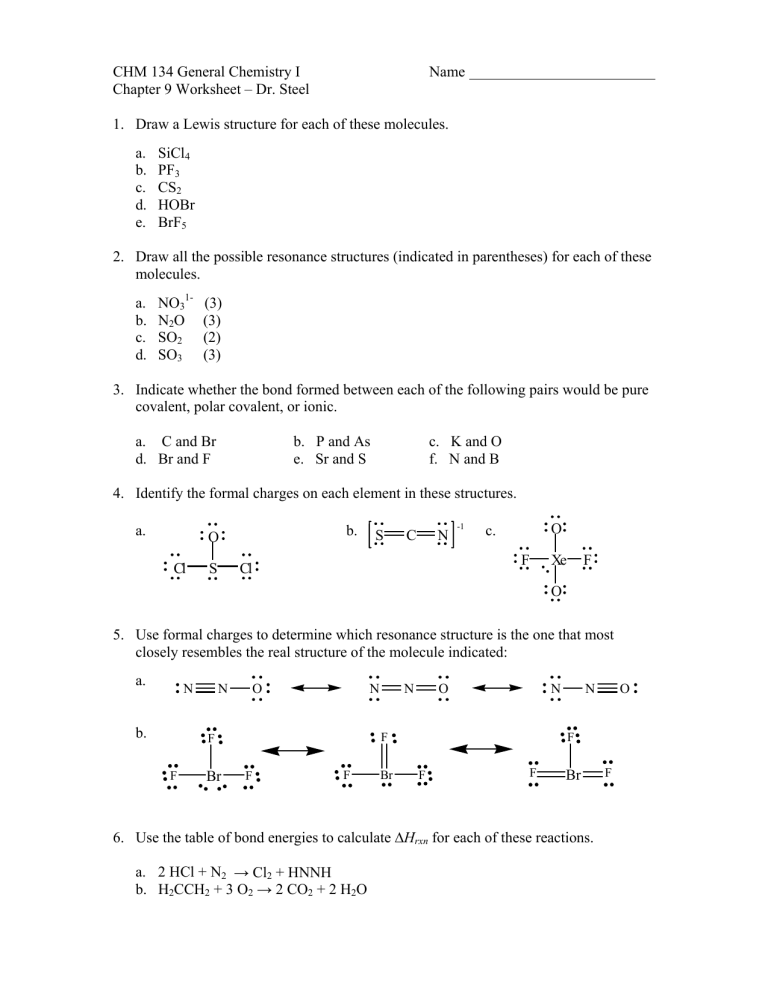
CHM 134 General Chemistry I Chapter 9 Worksheet – Dr. Steel Name 1. Draw a Lewis structure for each of these molecules. a. b. c. d. e. SiCl4 PF3 CS2 HOBr BrF5 2. Draw all the possible resonance structures (indicated in parentheses) for each of these molecules. a. b. c. d. NO31N2O SO2 SO3 (3) (3) (2) (3) 3. Indicate whether the bond formed between each of the following pairs would be pure covalent, polar covalent, or ionic. a. C and Br d. Br and F b. P and As e. Sr and S c. K and O f. N and B 4. Identify the formal charges on each element in these structures. a. b. O Cl S S C N -1 O c. F Cl Xe F O 5. Use formal charges to determine which resonance structure is the one that most closely resembles the real structure of the molecule indicated: a. N b. N O N Br O N F F F N F F Br N F F F Br 6. Use the table of bond energies to calculate ΔHrxn for each of these reactions. a. 2 HCl + N2 → Cl2 + HNNH b. H2CCH2 + 3 O2 → 2 CO2 + 2 H2O O F Answers: F Cl Cl 1. a. SiCl4 Si Cl P F b. PF3 F S C S c. CS2 Cl d. HOBr F e. H O F Br Br F -1 O 2. a. b. c. O N O N N O O S O O O d. O S F O N O O S O O S N N O O O O O c. ionic f. polar covalent c. Xe +2 O -1 F 0 5. a. first structure, O has -1 formal charge b. first structure, everything has a formal charge of 0 6. a. ΔHrxn = + 369 kJ N O b. pure covalent e. polar covalent b. S 0 C 0 N -1 -1 O O 3. a. pure covalent d. polar covalent 4. a. S +1 O -1 Cl 0 N N F -1 O O BrF5 b. ΔHrxn = 1291 kJ S O