The Sweetest Poison
advertisement
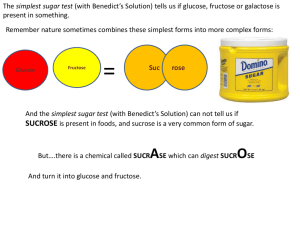
The Sweetest Poison When it comes to identifying the most common poison that we willingly use against ourselves, an amazing feat resulting in millions of deaths worldwide every year, there really is no contest. The perpetrator is as unlikely a candidate as any you might wish to name, and its unmasking is probably all the more horrifying because this substance has burrowed its way into our civilization like a parasite, draped in the false colors of comfort and familiarity. It’s whiter than heroin, sweeter than your fiancée, more soluble than the National Debt, and more pernicious than nicotine because, like a true demon, this little beauty comes in a million disguises and always dresses like a friend. Dr William Coda Martin was the first publicly to label sucrose a poison. Martin’s definition came about after he determined the classical definition of a poison was “…any substance applied to the body, which causes or may cause disease.” Refined sugar, or sucrose, is manufactured from cane and beet extract, which has had its proteins, vitamins and minerals removed to leave a white, crystalline substance devoid of any nutritional content, only offering empty calories. Sugars contained in natural, whole foods are easily metabolized by the body. Nature has ensured that fructose, for instance, obtained when we consume fruits, has the necessary vitamins and minerals accompanying it to allow this type of simple monosaccharide sugar to be converted efficiently into glucose (blood sugar) and fully metabolized by our bodies for energy. Vitamins and minerals, which accompany fructose, are essential for these complete assimilation and conversion processes to occur. Sucrose on the other hand, cannot metabolize completely in our bodies, resulting in the formation of toxic metabolites such as pyruvic acid and abnormal, unstable sugars containing five carbon atoms. These toxic by-products interfere with the respiration of cells, preventing the latter from acquiring sufficient oxygen to function correctly. These poisonous metabolites, in their free-radical or oxidation format, are constantly seeking to stabilize themselves by robbing our healthy cells of their available oxygen. This in turn degrades the cell and the cell dies. Extract from a report by Phillip Day as featured in Food Matters