General Chemistry II Name SOLUTIONS Exam 3 1. (10 points
advertisement
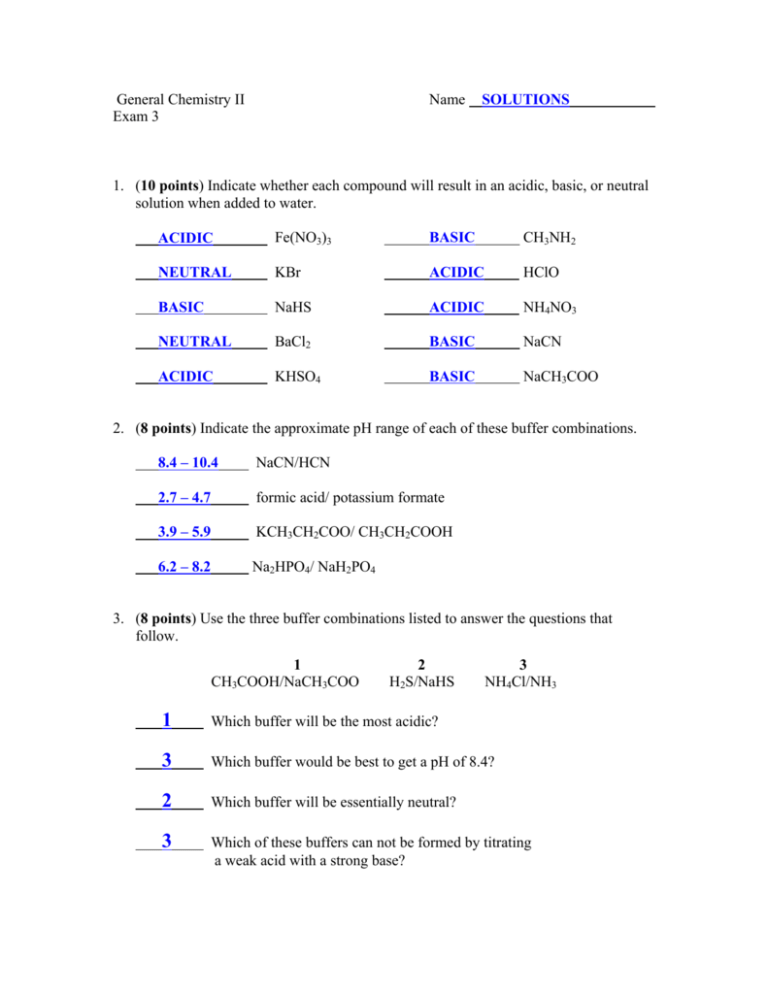
General Chemistry II Exam 3 Name SOLUTIONS 1. (10 points) Indicate whether each compound will result in an acidic, basic, or neutral solution when added to water. ACIDIC Fe(NO3)3 BASIC CH3NH2 NEUTRAL KBr ACIDIC HClO BASIC NaHS ACIDIC NH4NO3 NEUTRAL BaCl2 BASIC NaCN ACIDIC KHSO4 BASIC NaCH3COO 2. (8 points) Indicate the approximate pH range of each of these buffer combinations. 8.4 – 10.4 NaCN/HCN 2.7 – 4.7 formic acid/ potassium formate 3.9 – 5.9 KCH3CH2COO/ CH3CH2COOH 6.2 – 8.2 Na2HPO4/ NaH2PO4 3. (8 points) Use the three buffer combinations listed to answer the questions that follow. 1 CH3COOH/NaCH3COO 2 H2S/NaHS 3 NH4Cl/NH3 1 Which buffer will be the most acidic? 3 Which buffer would be best to get a pH of 8.4? 2 Which buffer will be essentially neutral? 3 Which of these buffers can not be formed by titrating a weak acid with a strong base? 4. (10 points) What mass of sodium hypochlorite (NaClO) should be added to 1.0 L of a 0.40 M solution of hypochlorous acid to produce a buffer whose pH is 7.75? [H O ] = 10 + − pH [ClO ] = 1.969 3 − = 10 −7.75 [HClO] = 1.778 × 10 −8 M + Ka [ClO ] = 1.969[HClO] − [H O ][ClO ] = − = 1.969(0.400) 3 [ClO ] = [HClO ] − [HClO ] Ka H 3O + [ = 0.7876 M moles NaClO = M ⋅ V = (0.7876 M )(1.0 L) ] 3.5 × 10 −8 1.778 × 10 −8 = 1.969 = = 0.7876 mol 0.7876 mol NaClO × 74.44 g NaClO = 58.6 g NaClO 1 mol NaClO 5. (12 points) Calculate the pH that results when 32.4 grams of sodium bicarbonate (NaHCO3) are dissolved in enough water to make 750. mL of solution. HCO3- + H2O ↔ OH- + H2CO3 [ ]0 0.514 0 0 ∆[] -x +x +x [ ]eq 0.514 x x 32.4 g NaHCO 3 × 1 mol NaHCO 3 = 0.3857 mol 84.008 g NaHCO 3 mol [HCO ] = 0.3857 = 0.514 M 0.750 L − 3 [H 2 CO 3 ][OH - ] x2 Kb = = = 2.4 × 10 −8 0.514 [HCO 3 ] x = 1.11 × 10 − 4 = [OH - ] pOH = −log[OH − ] = 3.954 pH = 14 - pOH = 10.046 6. (6 points) The Ksp of barium carbonate (BaCO3) is 2.6×10-9. How will the solubility of BaCO3 be altered, compared to its water solubility, (i.e. – increased, decreased, or unaffected) when it is dissolved in aqueous solutions of these compounds? UNAFFECTED NaOH INCREASED Na2SO4 UNAFFECTED CH3OH DECREASED BaCl2 INCREASED HBr INCREASED CH3CH2COOH 7. (12 points) The solubility product constant of silver sulfate, Ag2SO4, is 1.2×10-5. Determine the mass of Ag2SO4 that can be dissolved in 2.00 L of a 0.572-M solution of sodium sulfate, Na2SO4. [ ]0 Ag2SO4(s) ↔ 2 Ag+ --0 + SO40.572 ∆[] -S + 2S +S [ ]eq --- 2S 0.572 K sp = [Ag + ][Cl − ] 1.2 × 10 −5 moles Ag 2 SO 4 = M ⋅ V = (2S) (0.6) S = 0.00229 M = (0.00229 M )(2.00 L) = 0.00458 mol 2 0.00458 mol Ag 2 SO 4 × 311.87 g = 1.43 g Ag 2 SO 4 1 mole 8. (10 points) A buffer solution is prepared by mixing 350. mL of 0.368 M NaHSO3 and 150. mL of 0.622 M Na2SO3. Determine the pH of the solution. [NaHSO 3 ] = M 1 V1 = (0.368 M )(0.350 L) = 0.2576 M 0.500 L [Na 2SO 3 ] = M 1 V1 = (0.622 M )(0.150 L) = 0.1866 M 0.500 L V2 V2 HSO3- + H2O ↔ H3O+ + SO3-2 pH = p K a + log [base] [acid] = − log (6.2 × 10 −8 ) + log = 7.068 (0.1866) (0.2576) 9. (12 points) Determine the pH that results from mixing 21.6 mL of 0.225 M sodium hydroxide with 50.0 mL of 0.322 M hypochlorous acid, HClO, whose Ka = 3.5×10-8. HClO + OH- ClO- + H2O → mol 0.0161 0.00486 0 ∆ mol - 0.00486 -0.00486 + 0.00486 molfin [] VT = 71.6 mL 0.0112 0 0.00486 0.157 0.0679 pH = p K a + log [base] [acid] = − log (3.5 × 10 −8 ) + log (0.0.0679) (0.157) = 7.092 10. (12 points) Pyridine is a weak base whose Kb is 1.7×10-9. A 24.0-mL sample of a 0.180-M solution of pyridine is titrated with 0.140 M hydrochloric acid. Determine the pH of the titration solution after the addition of 18.5 mL of HCl. Pyr + H3O+ PyrH+ → mol 0.00432 0.00259 0 ∆ mol - 0.00259 - 0.00259 +0.00259 molfin [] VT = 42.5 mL 0.00173 0 0.00259 0.0407 + H2O 0.0609 -9 Kb = 1.7×10 ⇒ pKb = 8.770 ⇒ pKa = 5.230 [base] [acid] (0.0.407) = 5.230 + log (0.0609 = 5.055 pH = p K a + log