protein tyrosine kinases
advertisement

Phosphorylation
Inside:
Calcium/Calmodulin
Protein Kinases
Cyclic NucleotideRegulated Kinases
Cyclin-Dependent
Kinases
Mitogen-Activated
Protein Kinase
Pathway
PDK1-PKB/Akt
Pathway
Protein Kinase C
Protein Tyrosine
Kinases
Products
Kits
Enzymes
Antibodies
Activators
Inhibitors
®
®
sigma-aldrich.com
TA B L E O F C O N T E N T S
Calcium/Calmodulin Dependent Protein Kinases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-4
Overview, Tables and Product Lists
Cyclic Nucleotide-Regulated Kinases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-8
Overview, Tables and Product Lists
Cyclin-Dependent Kinases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9-11
Overview, Tables and Product Lists
MAP Kinase Pathway . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12-19
Overview, Tables and Product Lists
PDK1 - PKB/Akt Pathway . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .20-22
Overview, Tables and Product Lists
Protein Kinase C . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .23-28
Overview, Tables and Product Lists
Protein Tyrosine Kinases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .29-38
Overview, Tables and Product Lists
References/Abbreviations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .39-40
OVERVIEW
Phosphorylation is a ubiquitous cellular regulatory mechanism. It is a reversible, covalent modification of
a protein or lipid that serves to modify the activity of the phosphorylated molecule by inducing conformational changes within the molecule. This modification occurs either through the addition of phosphate
groups via the transfer of the terminal phosphate from ATP to an amino acid residue and/or by their
removal. The function of these post-translational modifications is to alter the substrate’s activity, subcellular
localization, binding properties or association with other proteins. Families of specialized molecules catalyze
the addition (kinases) or removal (phosphatases) of phosphate groups from proteins. Different classes of
protein kinases and phosphatases act specifically on serine/threonine residues, or tyrosine residues. An
important feature of kinases and phosphatases is that a single molecule is able to activate many substrate
molecules, thus allowing for amplification of the initial signal.
Kinases and phosphatases are of interest to researchers involved in drug discovery, because of their
broad relevance to health and disease. Cancer and other proliferative diseases, inflammatory diseases,
metabolic disorders and neurological diseases are among those in which protein phosphorylation plays an
important role. All signal transduction pathways are regulated, on some level, by phosphorylation, making
phosphorylation relevant to most, if not all, areas of cell signaling and neuroscience research.
®
®
Free calcium is a major second messenger in all cell types. One mechanism by which calcium ions exert
their effect is by binding to a 17 kDa protein, calmodulin (CaM). The binding of four calcium ions to
calmodulin changes its conformation and promotes its interaction with a number of other proteins,
including several classes of protein kinases that are activated by the calcium/CaM complex. A practical
way of classifying the calcium/CaM-dependent protein kinases is based on their substrate specificity: some
of these enzymes have only one substrate, and are designated as ‘dedicated’ calcium/CaM-dependent
protein kinases, while others have broad substrate specificity and are termed ‘multifunctional’ kinases.
The dedicated calcium/CaM-dependent protein kinases comprise three enzymes: phosphorylase kinase,
myosin light chain kinase and eEF2-kinase. Phosphorylase kinase, the first protein kinase to be identified,
Calcium/CaM-Dependent
Protein Kinases
CALCIUM/CALMODULINDEPENDENT PROTEIN KINASES
phosphorylates and activates glycogen phosphorylase, the enzyme that degrades glycogen. Phosphorylase
kinase is activated either by phosphorylation by cyclic AMP-dependent protein kinase or by the binding
of calcium/CaM. This mechanism of regulation is especially important in muscle where glycogen breakdown and muscle contraction are coordinated by the transient increase in cytoplasmic calcium levels.
Myosin light chain kinases (MLCK) are a group of enzymes that phosphorylate the regulatory light chain of
myosin. Smooth muscle MLCK induces smooth muscle contraction by increasing actin-activated myosin
ATPase activity. In contrast, striated muscle MLCK plays only a modulatory role in contraction by potentiating the effects of troponin-bound calcium on actin/myosin. In non-muscle cells, MLCKs are key factors
in the numerous processes which involve actin/myosin-based organelle movement or cell motility. eEF2kinase (also known as CaM-kinase III) phosphorylates eukaryotic elongation factor 2 (eEF2), a GTPase
necessary for the elongation step in protein translation. eEF2-kinase belongs to a separate class of
protein kinases that also includes myosin heavy chain kinases, and is distinct from the main family of
protein kinases with which they have no sequence similarity. Phosphorylation of eEF2 by eEF2-kinase
accounts for a calcium-dependent interruption of protein synthesis that may be responsible for a rapid
change in the nature of the mRNA being translated.
Multifunctional calcium/CaM-dependent protein kinases comprise three enzymes referred to as CaMkinases I, II and IV. CaM-kinase II (CaMKII) is an oligomer of probably 12 subunits that has unique properties and is also the most extensively studied. As is the case with other CaM-kinases, the activity of CaMKII
is inhibited by an autoinhibitory domain. This inhibition is alleviated by binding calcium/CaM which allows
autophosphorylation of the autoinhibitory domain. Once autophosphorylation has occurred, the presence
of calcium/CaM is no longer necessary and the enzyme becomes calcium/CaM-independent. Interestingly,
the oligomeric structure of CaMKII and the fact that autophosphorylation is a ‘trans’ reaction between
different subunits of the oligomer has important consequences. Autophosphorylation promotes calcium/CaM
trapping and occurs only when two adjacent subunits are bound to calcium/CaM. Thus, CaMKII is sensitive to the duration and frequency of calcium transients, and is capable of decoding the frequency of
calcium spikes. CaMKII may also remain active for some time while calcium levels return to normal,
thereby maintaining a transient ‘memory’ of neuronal activation. Its abundance in synaptic regions
and its actions on many proteins, including ion channels, make CaMKII an important contributor to
the processes of synaptic plasticity and the induction of LTP (Long Term Potentiation).
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
1
Calcium/CaM-Dependent
Protein Kinases
CALCIUM/CALMODULINDEPENDENT PROTEIN KINASES
CaMKI and CaMKIV are monomeric enzymes that share the common property of being activated by
calcium/CaM binding and by phosphorylation by a CaM-kinase-kinase (CaMKK). Together these kinases
are organized as a calcium/CaM-dependent protein kinase cascade. CaMKIV phosphorylates transcription
factors, including cAMP responsive element binding protein (CREB) and the associated CREB-binding
protein (CBP), and thus plays a major role in calcium-regulated gene transcription. CaMKK controls the
activity of both CaMKI and CaMKIV. CaMKK is also able to phosphorylate and activate PKB (Akt), and
thus exerts anti-apoptotic effects. Recently, a family of pro-apoptotic serine/threonine protein kinases has
been identified and termed death associated protein kinases (DAP-kinases). Two of these DAP-kinases
possess a CaM-binding domain and are activated by calcium/CaM.
Dedicated Calcium/CaM-Dependent Protein Kinases
2
Myosin Light Chain Kinase
(MLCK)
eEF2-Kinase
Phosphorylase Kinase (PHK)
Family
Immunoglobulin gene
superfamily
Eukaryotic protein kinase
superfamily
Regulatory enzyme of glycogenolysis
MW (kDa)
210 (non-muscle)
108 (smooth muscle)
95-105
α, β-125, γ-60
Domains
N-terminal actin-binding
domain, a central kinase
domain, and a C-terminal
myosin-binding domain
Putative calmodulin-binding
domain distal to the
catalytic domain
Two inhibitory domains in C-terminal
region; α, β-regulatory subunits,
β-barrel domains, δ-calmodulin family
subunit, calcium binding domain,
γ-catalytic subunit binding domain
Phosphorylation Sites
Thr803, Ser815
Ser365, Ser499
α-7 sites, β-3 sites
Tissue Distribution
Neurons, glia, heart,
platelets, muscles
Ubiquitous
Liver, muscle, kidney, heart,
testis, erythrocytes
Isoforms
MLCK 1, 2, 3a,
3b, and 4) (non-muscle)
None
α, β, γ, δ; each subunit
has several isoforms
Subcellular Localization
Plasma membrane (cytoskeleton) Cytoplasm
Cytoplasm
Species
Human, rabbit, mouse
Human, rat, mouse, rabbit,
chicken, yeast
Human, rabbit, mouse, yeast, fish
Other Names
MYLK
Eukaryotic elongation factor-2
kinase; CaM kinase III
Glycogen phosphorylase kinase
(GPK), Phk, adenosine triphosphate
(ATP)-phosphorylase β
Upstream Activator(s)
Calcium/CaM
p70S6K, p90rsk1
PKA, calcium/CaM
Downstream Activation
Myosin
eEF2
Glycogen phosphorylase
Disease States
None
Cardiac hypertrophy
α1-muscle glycogenosis glycogen
storage disease, hepatomegaly,
γ-cirrosis
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
Multifunctional Calcium/CaM-Dependent Protein Kinases
CaMKl
CaMKII
CaMKII-γ
CaMKIV
CaMKK
Family
Multifunctional
Multifunctional
Multifunctional
Multifunctional
Multifunctional
MW (kDa)
41
52-54
37
65-67
67
Domains
Autoinhibitory domain,
calcium/CaM
C-terminal binding
domain, N-terminal
hydrophobic residues
of calmodulin, activation loop contains Thr
in subdomain VIII
(phosphorylation
results in maximal
activity)
Autoinhibitory domain, Entirely catalytic and
calcium/CaM
regulatory domains
binding domain,
activation loop
Kinase catalytic
domain, calcium/
CaM-binding
domain, autoinhibitory domain,
activation loop contains Thr in subdomain VIII
(phosphorylation
results in maximal
activity)
Autoinhibitory domain,
RP-rich insert in
catalytic domain
between subdomains II
and III; C-terminus
folds back on itself;
unique N- and
C-terminal hydrophobic
pockets of calcium/
CaM anchor Trp444
and Phe459 of the
CaMKK peptide
Phosphorylation
Sites
Thr177, Thr286,
Thr305, Thr306,
Tyr267
Thr177, Thr286
Thr287
(autophosphorylation
site), Thr305, Thr306,
Tyr267
Ser12 and
Ser13, (autophosphorylation sites)
Thr196, Thr200
Thr108, Thr200,
Ser458, Ser74
Ubiquitous; highly
Islet cells, T-cells,
expressed in neurons, lymphoid organs
brain
Brain, neurons,
thymus, spleen,
testis, T-cells
Heart (different kinase
than in brain), brain,
thymus, spleen, T-cells
Tissue Distribution Heart
Isoforms
α
α, β, γ, δ C,
δ 2, δ 6, δ 11, δ 12
γ B, γ C, γ H, γ I, γ J,
γ K, γ L, γ M, γ N
α, β,
calspermin
α, β
Subcellular
Localization
Cytoplasm
Cytoplasm
Cytoplasm
Nucleus and
Cytoplasm
γ-Nucleus, cytoplasm,
β-nucleus
Species
Rodents, human, pig
Mammalian
Human, mouse
Pig, human, mouse
Rat, human
Other Names
Calcium/CaMdependent protein
kinase 1; CaMK1
Calcium/CaMdependent protein
kinase 2; CaM
kinase 2A
CaMKG;
Calcium/CaMdependent protein
kinase 2
Calcium/CaMCalcium/CaMdependent protein
dependent protein
kinase 4; CaMK4;
kinase kinase
Brain CaM kinase IV;
CaMK-Gr
Upstream
Activator(s)
CaMKK
Calcium/CaM
Calcium/CaM
CaMKK
Calcium/CaM
Downstream
Activation
Synapsin I, Synapsin II PLA2, EGFR
Not known
CREB, ATF-1, SRF
PKB, CaMKI, CaMKIV
Disease States
Human neuroblastoma, Behavioral abnorAutoimmune defects
cardiac hypertrophy
malities: long term
memory, fear response,
aggressiveness
Male infertility
Not known
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
Calcium/CaM-Dependent
Protein Kinases
CALCIUM/CALMODULINDEPENDENT PROTEIN KINASES
3
Calcium/CaM-Dependent
Protein Kinases
CALCIUM/CALMODULINDEPENDENT PROTEIN KINASES
Products Available from Sigma-RBI
Calcium/CaM-Dependent Protein Kinases
C 7331
Calmodulin-Dependent Protein Kinase II
Isolated from rat brain; serine/threonine protein kinase.
C 1360
Calmodulin Kinase II Inhibitor
Recombinant, rat. Full-length, with an N-terminal histidine tag
expressed in E. coli BL21 cells; CAM kinase II inhibitor.
w
Ne
Calcium/CaM-Dependent Protein Kinase Inhibitors
A 4308
Autocamtide 2-Related Inhibitory Peptide
Potent CaMKll inhibitor.
C-185
Cam Kinase II Inhibitor 281-302
CaMKll substrate antagonist.
C 2932
Chelerythrine chloride
Inhibits CaMK when used at millimolar concentrations.
G 1274
HA-1004 HCl
Effective CaMK II inhibitor; shown to be an intracellular calcium
antagonist.
I 2142
KN-62
Selective rat brain CamKll inhibitor.
K-112
KN-92
Does not inhibit CaMKll; negative control for KN-93.
K 1385
KN-93
Selective CaMKll inhibitor.
I 2764
ML-7
Selective MLCK inhibitor.
C 1172
ML-9
A cell-permeable MLCK inhibitor; also reported to inhibit agonistinduced Ca2+ entry into endothelial cells.
P 2277
Phosphodiesterase 3’:5’-Cyclic-Nucleotide
Activator
Isolated from bovine brain; Ca2+ binding protein required for
activation of cyclic nucleotide-dependent phosphodiesterase.
R 5648
Rottlerin
CaM kinase III inhibitor.
S 4400
Staurosporine
Isolated from Streptomyces sp.; potent phospholipid/calciumdependent protein kinase inhibitor.
S 2525
Syntide 2
Calcium/CaM-Dependent Protein Kinase Substrates
Calmodulin-dependent protein kinase substrate.
Antibodies to Calcium/CaM-Dependent Protein Kinases
C 6974
Anti-CaM Kinase II (α subunit)
Rabbit IgG fraction of antiserum. Applications: IP, IB
C-265
Monoclonal Anti-CaM Kinase II (α subunit)
(Clone 6G9) Mouse purified immunoglobin; Isotype IgG1.
Applications: IF, IB
P-247
Monoclonal Anti-Phosphorylated CaM
Kinase II (α subunit)
(Clone 22B1) Mouse purified immunoglobin; Isotype IgG1.
Applications: IF, IB
C 2851
Anti-CaM Kinase IV
Rabbit IgG fraction of antiserum. Applications: IHC, IB
C 7099
Anti-CaM Kinase Kinase
Rabbit affinity isolated antibody. Applications: IHC, IB
w
Ne
M 7905
4
Monoclonal Anti-Mysoin Light Chain Kinase (Clone K36) Mouse purified immunoglobin; Isotype IgG2b.
Applications: IP, IB
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
Cyclic AMP-dependent protein kinase (PKA or cAK) and cyclic GMP-dependent protein kinase (PKG or
cGK) transfer the γ-phosphate of ATP to serine and threonine residues of many cellular proteins. PKAs
are present in most cells and function as effectors of many cAMP-elevating first messengers such as
hormones and neurotransmitters. cGMP-elevating agents include nitric oxide, natriuretic peptides and
guanylin. In most tissues, PKGs are much less abundantly expressed than PKAs.
In the absence of its activating ligand cAMP, PKA exists as an inactive holoenzyme of two regulatory (R)
and two catalytic (C) subunits. Following an increase in intracellular cAMP, the (R)-subunits bind cAMP
resulting in the dissociation of the holoenzyme and the release of two free active catalytic (C)-subunits.
The active (C)-subunit phosphorylates peptide substrates containing the -R-R/K-X-S/T- substrate consen-
Cyclic NucleotideRegulated Kinases
CYCLIC NUCLEOTIDER E G U L AT E D K I N A S E S
sus amino acid sequence (although exceptions to this consensus sequence have been observed). The
holoenzymes can be anchored to specific compartments via interaction of their regulatory subunits with
specific PKA anchoring proteins (AKAPs).
In contrast to PKA, the regulatory and catalytic regions of the PKG enzyme are present in one polypeptide. Binding of cGMP to the two cGMP-binding sites is thought to release the autoinhibitory Nterminal domain from binding to the C-terminal catalytic domain, thus enabling substrate binding and
heterophosphorylation. The substrate consensus amino acid sequence for PKGs appears to require
multiple basic residues (consensus -R/K2-3-X-S/T-). However, in vitro, many substrate proteins can be
phosphorylated by both kinases. In addition to phosphorylating other proteins (heterophosphorylation),
each of the PKGs and type II PKA phosphorylate themselves (autophosphorylation). Within the cell, the
specific localization of the kinases and their substrates has been shown to restrict some of the possible
interactions suggested by in vitro data. PKG I is localized mainly in the cytoplasm and a number of PKG
anchoring proteins (GKAPs) have been identified. The PKG II enzyme is anchored to membranes via its
myristoylated N-terminus.
PKA has been shown to mediate the vast majority of cellular responses to the intracellular second
messenger cAMP in eukaryotes. Other effectors of cAMP are cAMP-regulated guanine nucleotide
exchange factors of small G proteins and cAMP-regulated ion channels. PKA I functions include the
inhibition of lymphocyte cell proliferation and immune response, mediation of long term depression in the
hippocampus, and sensory nerve transmission. PKA II mediates cAMP effects on neuronal gene expression and motor learning, on lipolysis and on sperm motility. The localization of PKA II via AKAPs to the
Golgi-centrosomal area in most cells, to receptors and ion channels, to the cytoskeleton and the nucleus
enables PKA II to regulate diverse cellular functions.
The second messenger cGMP has three major effector systems within the cell: cGMP-regulated ion
channels, cGMP-regulated phosphodiesterases and PKGs. PKG I mediates cGMP-induced smooth muscle
cell relaxation and inhibition of platelet aggregation. These effects correlate at least in part with an inhibition of calcium release from intracellular stores. In addition PKG I can inhibit cardiac myocyte contractility and has also been shown to regulate proliferation and gene expression in various cell types. PKG II
stimulates intestinal chloride secretion, inhibits renin release from juxtaglomerular cells, stimulates renal
calcium reabsorption and regulates endochondrial ossification.
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
5
Cyclic NucleotideRegulated Kinases
CYCLIC NUCLEOTIDER E G U L AT E D K I N A S E S
PKA/PKG
PKAI
PKAII
MW (kDa)
Catalytic subunit: 40; regulatory subunit: 42
Catalytic subunit: 40
Domains
Regulatory subunit RI and catalytic subunit C;
regulatory subunit comprises a dimer interaction
site, a hinge region (peptide inhibitory site) and
two cAMP-binding domains
Regulatory subunit RII and catalytic subunit C;
regulatory subunit comprises a dimer interaction
site, a hinge region (peptide inhibitory site) that
contains an autophosphorylation site, and two
cAMP-binding domains
Phosphorylation Sites
Catalytic: Ser10 (autophosphorylation site),
Thr197, Ser338
Catalytic: Ser10 (autophosphorylation site),
Thr197, Ser338
Tissue Distribution
Regulatory: brain, heart (RIα), lymphocytes (RIα);
Catalytic: ubiquitous (Cα1, Cβ1), brain (Cβ2, 3),
testis (Cαs, Cγ), lymphocytes (Cα1, Cβ2)
Regulatory: brain, heart (RIIα), lymphocytes (RIIα),
liver, rat (RIIβ); Catalytic: ubiquitous (Cα1, Cβ1),
brain (Cβ2, 3), testis (Cαs, Cγ), lymphocytes (Cα1, Cβ2)
Isoforms
RIα, RIβ; Cα1, Cαs, Cβ1, Cβ2, Cβ3, Cγ
RIIα, RIIβ; Cα1, Cαs, Cβ1, Cβ2, Cβ3, Cγ
Upstream Activator(s)
cAMP
cAMP
Downstream Activation
Phosphorylase kinase, glycogen kinase, CREB,
Raf-1, Rap1, RGS3
Phosphorylase kinase, glycogen kinase, CREB,
Raf-1, Rap1, RGS3
Subcellular Localization
Regulatory: cytoplasm
Regulatory: cytoskeletal structures, organelles,
membranes
Species
Human, mouse, rat, pig, C. elegans, yeast
Human, mouse, rat, pig, C. elegans, yeast
Other Names
cAMP-dependent protein kinase; cAPK;
protein kinase A
cAMP-dependent protein kinase; cAPK;
protein kinase A
Products Available from Sigma-RBI
Antibodies and Kits to Cyclic Nucleotides
6
A 0670
Anti-Adenosine 3’:5’-cyclic monophosphate Rabbit whole antiserum. Application: RIA
CA201
cAMP Enzyme Immunoassay Kit
Colorometric competitive immunoassay (EIA) for the quantitation
of cAMP; sufficient to perform 96 assays.
CA200
Direct cAMP Enzyme Immunoassay Kit
Colorometric competitive immunoassay (EIA) for the quantitation of
cAMP in samples treated with 0.1M HCl; useful for samples requiring minimal handling; sufficient to perform 96 assays.
G 4899
Anti-Guanosine 3’:5’-cyclic monophosphate Rabbit whole antiserum. Application: RIA
CG201
cGMP Enzyme Immunoassay Kit
Colorometric competitive immunoassay (EIA) for the quantitation of
cGMP; sufficient to perform 96 assays.
CG200
Direct cGMP Enzyme Immunoassay Kit
Colorometric competitive immunoassay (EIA) for the quantitation of
cGMP in samples treated with 0.1M HCl; useful for samples requiring minimal handling; sufficient to perform 96 assays.
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
PKGI
PKGII
155
155
Dimer interaction site, a hinge region (peptide inhibitory site)
that contains an autophosphorylation site, two cGMP-binding
domains and a catalytic domain
Dimer interaction site, a hinge region (peptide inhibitory site)
that contains an autophosphorylation site, two cGMP-binding
domains and a catalytic domain
Ser63 (autophosphorylation site), Ser79, Thr193, Thr317
Not known
Smooth muscle, platelets (Iβ), cerebellar Purkinje cells, lung (Iα),
lymphocytes (Iβ), cardiac myocytes (Iα), endothelial cells (not all)
Intestinal mucosa, kidney, brain, bone
PKGIα, PKGIβ
None
cGMP, cAMP, cIMP
cGMP, cAMP, cIMP
RGS3, RGS4
RGS3, RGS4
Cytoplasm, cytoskeletal membranes
Membranes
Human, Drosophila, sheep
Human, Drosophila, sheep
cGMP-dependent protein kinase; cGPK; protein kinase G
cGMP-dependent protein kinase; cGPK; protein kinase G
Cyclic NucleotideRegulated Kinases
CYCLIC NUCLEOTIDER E G U L AT E D K I N A S E S
Products Available from Sigma-RBI
Cyclic Nucleotides and Analogs
A 4137
Adenosine 3’:5’-cyclic monophosphate
Naturally occurring, highest purity (99-100%), PKA activator.
A 9501
Adenosine 3’:5’-cyclic monophosphate
Naturally occurring, purified (minimum 99%), PKA activator.
A 6885
Adenosine 3’:5’-cyclic monophosphate sodium Sodium salt of the naturally occurring PKA activator.
B 5386
8-Bromoadenosine 3’:5’-cyclic monophosphate Membrane-permeable cAMP analog that has greater resistance to
hydrolysis by phosphodiesterases than cAMP; activates PKA.
B 7880
8-Bromoadenosine 3’:5’-cyclic monophosphate Sodium salt of B 5386.
sodium
B 1381
8-Bromoguanosine 3’:5’-cyclic monophosphate Membrane-permeable cGMP analog which has greater resistance
sodium
to hydrolysis by phosphodiesterases than cGMP; activates cGMPdependent protein kinase.
A-165
Rp-cAMPS triethylamine
Rp-diastereomer of adenosine 3’,5’-cyclic phosphorothioate; specific
cAMP antagonist and competitive PKA inhibitor; binds weakly to
phosphodiesterase, so is resistant to hydrolysis by this enzyme.
A-166
Sp-cAMPS triethylamine
Sp-diastereomer of adenosine-3’,5’-cyclic monophosphothioate;
potent, membrane-permeable PKA activator that mimics the effects
of cAMP as a second messenger in numerous systems while being
resistant to cyclic nucleotide phosphodiesterases.
C 1081
8-Chloroadenosine 3’:5’-cyclic monophosphate Membrane-permeable cAMP analog; resistant to hydrolysis by
phosphodiesterases.
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
7
Cyclic NucleotideRegulated Kinases
CYCLIC NUCLEOTIDER E G U L AT E D K I N A S E S
Cyclic Nucleotides and Analogs (continued)
C 3912
8-(4-Chlorophenylthio)adenosine 3’:5’-cyclic Membrane-permeable cAMP analog; selective PKA activator; inhibits
monophosphate
cGMP-dependent phosphodiesterase and, at higher concentrations,
inhibits cAMP-dependent phosphodiesterase.
C 5438
8-(4-Chlorophenylthio)guanosine 3’:5’-cyclic Membrane-permeable cGMP analog that does not affect cGMPmonophosphate
regulated phosphodiesterase; more potent cGMP analog than 8-BrcGMP due to greater membrane permeability and a higher resistance to hydrolysis by phosphodiesterase; selective PKG activator.
C-240
Rp-8-[(4-chlorophenyl)thio]-cGMPS
PKGα inhibitor; more cell-permeable than Rp-cGMPS.
D 0260
N6,2’-O-Dibutyryladenosine
3’:5’-cyclic
Cell-permeable cAMP analog that activates PKA.
D 0627
N6,2’-O-Dibutyryladenosine 3’:5’-cyclic
monophosphate sodium
Cell-permeable cAMP analog that activates PKA.
D 3510
N2,2’-O-Dibutyrylguanosine 3’:5’-cyclic
monophosphate sodium
Cell-permeable cGMP analog that activates PKG; has been shown
to increase intracellular calcium concentration in neurons and
hepatocytes.
G 7504
Guanosine 3’:5’-cyclic monophosphate
PKG stimulator.
G 6129
Guanosine 3’:5’-cyclic monophosphate sodium PKG stimulator.
G-135
Rp-cGMPS
Rp-diastereomer of guanosine 3’,5’-cyclic monophosphothioate;
PKGα inhibitor.
G-136
Sp-cGMPS
Sp-diastereomer of guanosine 3’5’-cyclic monophosphorothioate,
PKGα inhibitor.
P 2645
Protein kinase, catalytic subunit
Source: bovine heart. Catalytic subunit of PKA; does not require
cAMP for activity. Purified from P 5511.
P 5511
Protein kinase, 3’:5’-cyclic AMP-dependent,
bovine heart
Source: bovine heart. Phosphorylating activity is elevated to at least
10-fold in the presence of cAMP.
A 8186
Arg-Lys-Arg-Ala-Arg-Lys-Glu
PKG inhibitor.
C 2932
Chelerythrine chloride
Inhibits PKA at micromolar concentrations.
I 7016
H-7 DiHCl
PKA inhibitor.
I 6891
H-7
PKA inhibitor.
H-122
H-8 HCl
PKA and PKG inhibitor.
B 1427
H-89 HCl
Selective, potent PKA inhibitor.
G 1274
HA-1004 HCl
PKA and PKG inhibitor.
K 3761
KT5720
Specific, cell-permeable PKA inhibitor; no significant effect on PKC,
PKG or myosin light chain kinase (MLCK).
I 2764
ML-7
Inhibits PKA at micromolar concentrations.
C 1172
ML-9 HCl
Inhibits PKA at low micromolar concentrations.
A 3317
Malantide
High affinity PKA substrate.
K 1127
Kemptide
PKA substrate.
monophosphate sodium
Cyclic Nucleotide-Regulated Kinases
Cyclic Nucleotide-Regulated Protein Kinase Substrates
w
Ne
Cyclic Nucleotide-Regulated Protein Kinase Activators
Antibodies to Cyclic Nucleotide-Regulated Kinases
P 2729
8
Anti-PKA
Rabbit, affinity isolated antibody. Application: IF
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
Cyclin-dependent kinases (CDKs) are serine/threonine kinases that are crucial for cell cycle progression
and function as kinases only when complexed with cyclins. Within the complexes, the cyclin molecule
serves a regulatory role, whereas the CDK has a catalytic activity. To date, nine CDKs, referred to as
CDK1-CDK9, and 11 cyclins have been identified in human.
The structure of CDK2 consists of an amino-terminal lobe rich in β-sheets and a larger, mostly α-helical,
carboxy-terminal lobe. The ATP binding site is located in a deep cleft between the two lobes that contains the conserved catalytic residues. Crystallographic studies have shown the important influence
cyclin binding has on CDK2, forcing the kinase into an active conformation. First, the T-loop, which
blocks substrate access to monomeric CDK2, is located outside the catalytic cleft after cyclin A binds.
Cyclin-Dependent
Kinases
CYCLIN-DEPENDENT KINASES
This allows the activating phosphorylation of Thr160 by CDK7/cyclin H/MAT1. The second conformational change induced by cyclin binding is found within the ATP-binding site where a reorientation of
the amino acid side chains allows the alignment of the triphosphate of ATP necessary for phosphate
transfer. The strong sequence homology between the catalytic domains of different CDKs suggests that
their tertiary structures will be similar.
Progression through the G1, S, G2 and M phases of the cell cycle is directly controlled by CDKs. In earlymid G1, extracellular signals modulate the activation of CDK4 and CDK6 associated with D-type cyclins.
These complexes phosphorylate and inactivate the retinoblastoma protein pRb, resulting in the release
of the E2F and DP1 transcription factors that control the expression of genes required for the G1/S transition and S phase progression. The CDK2/cyclin E complex, that is responsible for the G1/S transition,
also regulates centrosome duplication. During S phase, CDK2/cyclin A phosphorylates different substrates
allowing DNA replication and the inactivation of G1 transcription factors. Around the S/G2 transition,
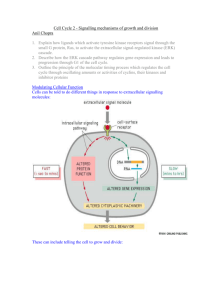
IR
ATM
Chk1
p53
p21
Cdc25C
Cdc25C
P
14-3-3
CAK
P P
Cdc2
P
DNA damage to Ataxia telangiectasia mutated gene (ATM) following exposure to γ-irradiation (IR)
prevents both phosphorylation and dephosphorylation of cdc2 through Chk1 and p53, respectively.
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
9
Cyclin-Dependent
Kinases
CYCLIN-DEPENDENT KINASES
CDK1 associates with cyclin A. Later, CDK1/cyclin B appears and triggers the G2/M transition by phosphorylating a large set of substrates. Phosphorylation of the anaphase promoting complex (APC) by
CDK1/cyclin B is required for transition to anaphase and completion of mitosis. These successive waves
of CDK/cyclin assemblies and activations are tightly regulated by post-translational modifications and
intracellular translocations. They are coordinated and dependent on the completion of previous steps,
through so-called “checkpoint” controls. Some CDKs directly regulate transcription. CDK7/cyclin H/MAT1
is a component of the transcription factor TFIIH. CDK9/cyclin T is a component of the positive transcription elongation factor P-TEFb. It is responsible for the Tat-associated kinase activity involved in the HIV-1
Tat transactivation.
CDK5 is the only tissue specific CDK and is found only in neuronal cells. Its activity is important for outgrowth of neurites and neuronal development, for myogenesis and for somite organization in embryos.
An interesting aspect of CDK5 is the nature of its associated regulatory subunits, p35 or its proteolytic
cleavage product, p25. The predicted structure of p35/p25 shows a similar fold to that of cyclins, which
explains the efficient activation of CDK5. Conversion of p35 to p25 leads to constitutive activation of
CDK5 and alteration of its cellular localization. CDK5/p25 expression in cultured primary neurons triggers apoptosis. Considerable evidence indicates links between CDK5 activity and the cytoskeletal abnormalities and neuronal death observed in Alzheimer’s disease.
Cyclin-Dependent Kinases
10
CDK1
CDK2
CDK3
CDK4
MW (kDa)
34
33
35
34
Domains
Regulatory subunit,
catalytic subunit contains
an activation segment,
Thr in loop is conserved
Regulatory subunit,
catalytic subunit contains
an activation segment,
Thr in loop is conserved
Regulatory subunit,
catalytic subunit contains
an activation segment,
Thr in loop is conserved
Regulatory subunit,
catalytic subunit contains
an activation segment,
Thr in loop is conserved
Phosphorylation Sites
Thr14, Tyr15, Thr160,
Thr161, Ser277
Thr14, Tyr15, Thr160,
Ser277
Thr14, Tyr15, Thr160
Tyr17, Tyr172
Tissue Distribution
Ubiquitous
Ubiquitous
Ubiquitous
Ubiquitous
Complex Partner
Cyclin B1,
Cyclin B2
Cyclin A,
Cyclin D,
Cyclin E
Cyclin E2
Cyclin D1,
Cyclin D2,
Cyclin D3
Upstream Activator(s)
CAK, Myt1(Wee1), MyH,
Cdc25
CAK, MyH
Cyclin E2
cdc25B, CAK
Downstream
Activation
Histone H1, RNAP II
Rb, Histone H1
p27, myc, Histone H1
Rb, MyoD
Subcellular
Localization
Nucleus
Cytoplasm, nucleus
Cytoplasm, nucleus
Cytoplasm, nucleus
Species
Multicellular organisms,
yeast
Multicellular organisms,
yeast
Multicellular organisms,
yeast
Multicellular organisms
Other Names
p34cdc2
p33 protein kinase
None
None
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
CYCLIN-DEPENDENT KINASES
Cyclin-Dependent Kinases and Phosphatases
C 7484
CDC25A, Active
Recombinant; full-length human expressed in E. coli; member of Cdc25 family of
tyrosine phosphatases which inhibit CDKs.
C 7609
CDC25B, Active
Recombinant; full-length human expressed in E. coli; member of Cdc25 family of
tyrosine phosphatases which inhibit CDKs.
W 4387
Wee 1, Active
Recombinant; full-length rat expressed in E. coli; phosphorylates and inactivates CDK2.
I 0404
Indirubin 3’-monoxime
CDK inhibitor that exhibits antiproliferative activity leading to G2/M arrest in many cell
lines and G1/S arrest in Jurkat cells.
O 0886
Olomoucine
Purine derivative that inhibits CDK and induces G1 arrest.
A 3145
Apigenin
Plant flavinoid; inhibits cell proliferation by arresting the cell cycle at the G2/M phase.
R 7772
Roscovitine
Potent, selective CDK inhibitor.
Cyclin-Dependent
Kinases
Products Available from Sigma-RBI
Cyclin-Dependent Kinase Inhibitors
w
Ne
Cyclin-dependent kinase antibodies are also available.
Please see our Web site for information on the following products:
C
C
C
C
4710
7464
7339
7214
Anti-Cyclin
Anti-Cyclin
Anti-Cyclin
Anti-Cyclin
A Monoclonal
D1 Monoclonal
D2 Monoclonal
D3 Monoclonal
C
C
C
C
4210
8831
5588
4976
Anti-Cyclin
Anti-Cyclin
Anti-Cyclin
Anti-Cyclin
A
C 5226 Anti-Cyclin G
B1
C 5351 Anti-Cyclin H
D1
C 0231 Anti-Phospho-CDK1 (pThr14/pTyr15)
E Monoclonal
CDK5
CDK6
CDK7
CDK8
CDK9
33
37
39
53
43
Regulatory subunit,
catalytic subunit contains
an activation segment,
Thr in loop is conserved
Regulatory subunit,
catalytic subunit contains
an activation segment,
Thr in loop is conserved
Regulatory subunit,
catalytic subunit contains
an activation segment,
Thr in loop is conserved
Regulatory subunit,
catalytic subunit contains
an activation segment,
Thr in loop is conserved
Regulatory subunit,
catalytic subunit contains
an activation segment,
Thr in loop is conserved
Thr14, Tyr15, Ser159
Thr177
Thr170, Tyr176, Ser164,
Ser170
Not known
Ser2, Ser5
Brain, neuronal cells
Ubiquitous
Ubiquitous
Ubiquitous
Ubiquitous
p35
Cyclin D1,
Cyclin D2,
Cyclin D3
Cyclin H
Cyclin C
Cyclin T,
Cyclin K
Phosphorylation indepen- CAK
dent activation by p35/p25
(nck5a) and p39 (nck5ai)
cdk2-Cyclin A,
cdk1-cyclin B
Not known
Not known
Histone H1, τ, MAP2,
NF-H, NF-M, DARPP-32
Rb
CDK1, CDK2, CDK4,
RNAPII
RNAPII
RNAPII, Rb,
myelin basic protein
Axon
Nucleus, cytoplasm
Nucleus
Nucleus, cytoplasm
Non-nucleolar nucleoplasm
Multicellular organisms
Multicellular organisms,
yeast
Multicellular organisms,
yeast
Multicellular organisms,
yeast
Multicellular organisms,
yeast
None
τPKII
CAK, STK1, p39 MO15
None
P-TEFB (CDK9/Cyclin T)
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
11
MAP Kinase
Pathway
M A P K I N A S E PAT H W AY
The mitogen-activated protein kinase (MAPK) family consists of both stress-activated (SAPK) and
mitogen-activated (MAPK) protein kinases. Together they form a network of signal transduction cascades
that mediate cellular responses to a diverse range of stimuli, including growth factors, chemical or
osmotic stress, irradiation, bacterial infection and proinflammatory cytokines. Each MAPK is activated
by dual phosphorylation on a Thr-Xaa-Tyr motif by upstream kinases, referred to as MAPK kinases or
MEKs (MKKs). MEKs are, in turn, activated by MAP3K (MKK kinases, MKKKs), over 30 of which have
been described. However, the details of how they are activated or which MAP3K really activates which
MEK in vivo is still poorly understood. MAPK and SAPK cascades frequently function as multi-protein
complexes in which the different components are assembled on a scaffold protein and/or by specific
protein-protein interactions, thereby increasing the speed and specificity of the cascade. MAPKs phosphorylate their substrates on serine or threonine residues which preceed a proline, but their specificity in
vivo is further enhanced by the presence of distinct docking sites that facilitate interaction with substrates. To date, 12 different MAPK family members have been identified in mammalian cells, and
homologs are found in all eukaryotic cells. Information about the gene nomenclature of mammalian
MAPKs can be obtained from http://www.gene.ucl.ac.uk/users/hester/prkm.html.
The most studied cascades in mammalian cells are the classical ERK1/2, p38 (SAPK2) and c-jun Nterminal kinase or JNK (SAPK1) cascades. The classical MAPK cascade, comprised of extracellular signal
regulated kinase 1 (ERK1) and ERK2, is activated by mitogens and growth factors, and plays an important role in the control of cell growth and differentiation. However, its inappropriate activation can lead
to cell transformation and cancer. ERK5 is also activated in vivo by mitogens and has been suggested to
be important for epidermal growth factor (EGF)-induced cell proliferation. ERK3 and ERK7 are more
Mitogen-Activated Protein Kinase Kinases (MEKs, MKKs)
MEK1
12
MEK2
MEK3
MW (kDa)
43.5
Domains
11 Conserved kinase domains;
proline rich segment
11 Conserved kinase domains;
proline rich segment
11 Conserved kinase domains
Phosphorylation Sites
Ser218, Ser222
Ser218, Ser222
Ser189, Thr193
Tissue Distribution
Ubiquitous; high levels in
murine brain
Ubiquitous; highest levels in
skeletal muscle
Skeletal muscle
Isoforms
MEK1, MEK1b
None
MEK3, MEK3b
Upstream Activator(s)
Raf, MAP3K3, MAP3K2
Raf
MAP3K5, MAP3K7,
MAP3K4, TAK1, Tao1, Tao2
Downstream Activation
ERK1, ERK2
ERK1, ERK2
p38
Subcellular Localization
Cytoplasm
Cytoplasm
Cytoplasm
Species
Eukaryotes
Eukaryotes
Eukaryotes
Other Names
MAP2K1, MKK1,
MAPKK1, PRKMK1
PRKMK2, MKK2,
MAPKK2, MAP2K2
PRKMK3, MKK3, MAPKK3,
MAP2K3, SKK2
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
recently described MAPKs. ERK7 appears to be constitutively phosphorylated on its Thr-Xaa-Tyr motif,
and its substrates and activators are unknown. ERK3 is unusual in that the Thr-Xaa-Tyr phosphorylation
motif is replaced by Ser-Xaa-Glu.
Stress
Growth Factors
MAP4K
PAK
GTP
?
?
PL C
Src
Rac
?
Y
P
JAK
MAP3K
MEKK
?
P Grb2 Sos
R as
14-3-3
MAP Kinase
Pathway
M A P K I N A S E PAT H W AY
PKC
GTP
?
P I3K
Raf-1
ST AT
B-Raf
Mos
MAPKK
MEK5
MKK3/4
JUNKK
(SEK ? )
ERK5
HOG
(p38)
JNK
(S AP K)
AKT
MAP KK
(MEK)
S6K
MAPK
MAPK
MAPK
(ERK)
(ERK)
Cytosolic
targets
MAPKAP
Kinase 2
MAPKAP
P90 rsk
ATF 2
Jun
GSK-3
Fos
Elk
Nuclear
targets
Activation of ERK, JNK and p38 pathways following stimulation by stress or growth factors.
MEK4
MEK5
MEK6
MEK7
47
11 Conserved kinase
domains
11 Conserved kinase domains;
long N-terminal sequence
11 Conserved kinase domains
11 Conserved kinase domains
Ser254, Thr258,
Ser257, Thr261
Ser311, Thr315
Ser207, Thr211
Ser206, Thr210
CNS, liver
Heart, skeletal muscle
MEK6: skeletal muscle; MEK6b:
heart, pancreas, liver, skeletal muscle
Ubiquitous; highest levels in
skeletal muscle
None
None
MEK6, MKK6b
MEK7α, MEK7β, MEK7γ
MAP3K5, MAP3K7,
SPRK, MAP3K1, MAP3K2,
TAK1, MLK2, MLK3, DLK
MAP3K3, MAP3K2
MAP3K5, MAP3K4, MAP3K7,
MAP3K4, TAK1
MAP3K1, MLK2, MLK3, DLK,
MAP3K3
JNK
ERK5
p38
JNK
Cytoplasm
Cytoplasm
Cytoplasm
Cytoplasm
Eukaryotes
Eukaryotes
Eukaryotes
Eukaryotes
SEK1, SKK1, PRKMK4,
MKK4, MAPKK4,
MAP2K4, JNKK1
PRKMK5, MKK5, MAPKK5,
MAP2K5
PRKMK6, MKK6, MAPKK6,
MAP2K6, SKK3
PRKMK7, MKK7, MAPKK7,
MAP2K7, JNKK2, SKK4
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
13
M A P K I N A S E PAT H W AY
MAP Kinase
Pathway
Mitogen-Activated Protein Kinases (MAPKs)
ERK1/2
JNK
p38
p38γ
MW (kDa)
44/42
46/54
38
38
Domains
Kinase catalytic domain,
TEY motif, activation
loop between
subdomains VII and VIII
Kinase catalytic domain,
TPY motif, activation loop
between subdomains
VII and VIII
Kinase catalytic domain,
TGY motif, activation
loop between subdomains
VII and VIII
Kinase catalytic domain,
TGY motif, activation
loop between subdomains
VII and VIII
Phosphorylation
Sites
ERK1: Thr202, Tyr204
ERK2: Thr185, Tyr187
Thr183, Tyr185, Thr404,
Ser407
Thr180, Tyr182
Thr183, Tyr185
Tissue
Distribution
Ubiquitous
Ubiquitous; JNK3 restricted
to brain, heart and testis
Ubiquitous
Low expression in most
tissues, very high levels
in skeletal muscle
Isoforms
ERK1, ERK2
JNK2 (SAPK 1a, SAPKα),
JNK3 (SAPK1b, SAPK-β),
JNK1 (SAPK1c, SAPK-γ)
p38α, p38β
None
Upstream
Activator(s)
MEK1
MEK4, MEK7
MEK3, MEK6
MEK6
Downstream
Activation
MAPKAP-K1/2,
MSK13, MNK, Elk1
MAPKAP-K1, MAPKAP-K3,
ATF2, Elk1, JunD, c-Jun
MAPKAP-K2, MAPKAP-K3, PRAK, NFκB, ATF2
MNK, MSK, ATF2, MEF2C
Subcellular
Localization
Cytoplasm, nucleus
Cytoplasm, nucleus
Cytoplasm, nucleus
Plasma membrane (muscle)
Species
Eukaryotes
Eukaryotes
Eukaryotes
Eukaryotes
Other Names
MAPK
SAPK1
SAPK2a, SAPK2b, p40,
CSBP, Mxi2
SAPK3, ERK6
The JNK (SAPK1) cascade is activated by cellular stress, bacterial infection and proinflammatory
cytokines, and results in the phosphorylation of AP1 transcription factors, such as c-Jun. The p38
(SAPK2) cascade is activated by similar stimuli to JNK. p38γ has been shown to bind to, and to colocalize with, α1-syntrophin by virtue of the interaction of its C-terminus with the PDZ domain of α1syntrophin. Nothing is yet known about the function of p38δ.
The MAPK family kinases phosphorylate MAPK-activated protein (MAPKAP) kinases. MAPKAP kinases
can be subdivided into two groups: those comprising two kinase domains in a single polypeptide, and
those with a single kinase domain. Both groups contain a C-terminal docking site that interacts with the
activator, thereby permitting phosphorylation and activation of the MAPKAP kinases.
The two kinase domain enzymes are MAPKAP-K1 (also called p90 Rsk), and mitogen and stress activated
protein kinase (MSK). MAPKAP-K1 is implicated in the regulation of several processes including cell
survival, gene transcription and the control of meiosis. Mutations in the human MAPKAP-K1b isoform
are linked to Coffin Lowry syndrome, a disease associated with mental retardation and growth defects.
MSK can be activated by either ERK1/ERK2 in response to mitogens and growth factors or by p38
following exposure to cellular stresses, proinflammatory cytokines and infection. Its N-terminal kinase
domain is 54% identical and its C-terminal kinase domain is 44% identical to the corresponding
14
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
p38δ
ERK3
ERK5
ERK7
42
62/100
95
61
Kinase catalytic domain,
TGY motif, activation loop
between subdomains
VII and VIII
Kinase catalytic domain,
SEG motif, activation loop
between subdomains
VII and VIII
Large C-terminal domain,
loop-12 sequence
TEY motif, activation
loop
Putative ATP binding
site, TEY activation motif:
threonine-glutaminetyrosine activation sequence
within subdomain VIII
Thr180, Tyr182
Ser189
Thr218, Tyr220
Thr175, Tyr177
Low expression in most
tissues, highest levels in
pancreas, testis
Ubiquitous
Ubiquitous
Low expression in most
tissues, high levels in
testis
None
ERK3, ERK3-related kinase
None
None
MEK6
ERK3 kinase
MEK5
CLIC3
NFκB, ATF2, eEF2K
MAP2
MEF2A/C
Not known
Cytoplasm
Nucleus
Cytoplasm, nucleus
Cytoplasm, nucleus
Eukaryotes
Eukaryotes
Eukaryotes
Eukaryotes
SAPK4
None
BMK1
None
MAP Kinase
Pathway
M A P K I N A S E PAT H W AY
domains of MAPKAP-K1. All the phosphorylation sites in MAPKAP-K1 are conserved in MSK, suggesting
an analogous mechanism of activation. However, in contrast to MAPKAP-K1, PDK1 is not required for
the activation of MSK, implying that the phosphorylation taking place in the activation loop of the
N-terminal kinase domain must be catalyzed by another protein kinase (perhaps MSK itself). The
Drosophila kinase JIL-1, an MSK1 homolog, has been localized to decondensed regions of chromosomes, suggesting a role in transcriptional regulation.
The MAPKAP kinases comprising a single kinase domain are MAPKAP-K2, MAPKAP-K3, MAPKAP-K5
(also called p38-regulated/activated kinase or PRAK) and MAPK-integrating kinase (MNK). MAPKAP-K2
is also involved in controlling production of the proinflammatory cytokines, tumor necrosis factor, interleukin 6 and interferon γ, at a post-transcriptional level. This may result from the ability of MAPKAP-K2
to regulate the stability and/or translation of the mRNAs containing AU rich regions. Little is known
about the physiological role(s) of MAPKAP-K5.
One substrate of MNK1 is the eukaryotic translation initiation factor 4E (eIF4E). Phosphorylation of this
protein increases its affinity for the 5’ cap of the mRNA, thereby promoting translation. MNK1 may also
be the protein kinase that mediates the thrombin-induced phosphorylation of a residue near the Cterminus of phospholipase A 2 in platelets, which contributes to the activation of this enzyme.
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
15
M A P K I N A S E PAT H W AY
MAP Kinase
Pathway
MAPK-Activated Protein Kinases (MAPKAP Kinases)
MAPKAP-K1
MAPKAP-K2
MAPKAP-K3
MW (kDa)
90
47-55
42
Domains
2 Kinase domains, 1 at
C terminus; linker region
between the 2 kinase domains,
N-terminal domain, activation
loop within N terminal domain
1 Kinase domain, 2 putative
SH3 domains, phosphorylated Thr
adjacent to nuclear localization
signal
1 Kinase domain, putative
N-term SH3 domain, 2 MAP
kinase phosphorylation site
motifs, putative ATP binding site
and nuclear localization signal
Phosphorylation
Sites
Ser222, Thr360, Ser364,
Ser381, Thr574, Ser733
Thr25, Thr222, Ser272
Thr201, Thr313
Tissue Distribution
Skeletal muscle
Ubiquitous
Ubiquitous; high levels in heart
and skeletal muscle
Isoforms
MAPKAP-K1a (RSK1),
MAPKAP-K1b (RSK2),
MAPKAP-K1c (RSK3),
MAPKAP-K1d (RSK4)
None
None
Upstream Activator(s)
ERK1, ERK2, MEK1, JNK
p38, ERK1, ERK2
p38, JNK
Downstream
Activation
CREB, MSK1, BAD
HSP27, CREB, α, β-crystallin,
ATF-2, SRF, E47, 5-LO lymphocyte
specific protein (LSP-1)
HSP27, CREB, E47
Subcellular
Localization
Cytoplasmic, nuclear
Cytoplasmic, nuclear
Cytoplasmic, nuclear
Species
Eukaryotes
Eukaryotes
Eukaryotes
MK2
3PK
Other Names
RSK,
p90Rsk
MAPK-Activated Protein Kinases (MAPKAP Kinases)
16
MAPKAP-K5
MSK
MNK
MW (kDa)
54
90
Domains
N-Terminal regulatory
domain, C-terminal
kinase domain
2 Kinase domains: 1 at C terminus,
linker region between them,
N-terminal domain, activation loop
within N-terminal domain
1 Kinase domain,
C-terminal Ert-interacting
domain
Phosphorylation
Sites
Ser93, Thr186, Ser212,
Ser214, Thr182
Ser360, Thr581
Thr197, Thr202
Tissue Distribution
Brain, heart, skeletal muscle,
lung, kidney, pancreas, placenta
Brain, muscle, placenta
Ubiquitous; high levels in
skeletal muscle, low levels in brain
Isoforms
None
MSK1, MSK2
MNK1, MNK2
Upstream Activator(s)
MAPKAP-K2, p38
ERK1/2, p38, MAPKAP-K3
Erk1/2, p38
Downstream
Activation
HSP25/27
CREB, eIF-4E, ATF-1
eIF-4E
Subcellular
Localization
Cytoplasm, nucleus
Nucleus
Cytoplasm
Species
Eukaryotes
Eukaryotes
Eukaryotes
Other Names
PRAK
RSK-B
None
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
M A P K I N A S E PAT H W AY
MAP Kinases
B 1307
B-Raf, Active
Recombinant full length human B-Raf expressed in Sf9 cells.
Application: Protein kinase assay.
C-Jun (1-169)-GST, Soluble
Truncated human c-Jun sequence-expressed in E. coli; substrate for SAPK1/JNK2.
MAP Kinase Kinase 6/SKK3,
Active
human, recombinant
N-terminal Mal-E-tagged MKK6/SKK3 fusion protein, expressed in E. coli.
Applications: Activation of p38α followed by phosphorylation of MBP.
MEKK1
mouse, recombinant
MEKK expressed in E. coli. Applications: Assay of MAPK2 activation and
MBP phosphorylation via a MEKK-dependent kinase cascade.
MEK2, Active
human, recombinant
MEK2 fused with GST at the N-terminus (71kDa fusion protein) expressed
in E. coli. Application: Coupled protein kinase assay.
w
Ne
C 5859
w
Ne
M 5814
w
Ne
M 6939
w
Ne
M 7064
w
Ne
M 3172
Mitogen-Activated Protein Kinase Rat recombinant expressed in E. Coli. Application: Kinase assays.
M 1689
MKK4/SKK1, Active
mouse, recombinant
MKK4, amino acids 35-357 fused to an N-terminal GST-tag expressed in
E. coli. Applications: MKK4 dependent activation of JNK1 or JNK2 and
phosphorylation of ATF2.
MKK7 α1, Active
recombinant
GST fusion protein encoding the last 333 residues of human MKK71
expressed in E. coli. Applications: protein phosphorylation assay.
MKK7 β1, Active
human, recombinant
MKK7β1, corresponding to amino acids 2-419, containing a GST-tag and a
Flag-tag™ expressed in E. coli. Applications: MKK7β1-dependent activation
of JNK1 or JNK2 followed by phosphorylation of ATF2.
MSK1, Active
human, recombinant
Full length expressed in Sf9 cells; activated by stress stimuli and
growth factor/phorbol ester.
p38-Regulated/Activated Protein
Kinase, human, recombinant
Full length protein expressed in Sf9 cells. Applications: phosphorylation of
PRAK substrate peptide.
Raf-1 (δ 1-306), Active
human, recombinant
N-terminal, GST-tagged truncated Raf-1 enzyme lacking residues 1-306;
expressed in Sf9 cells. Applications: Coupled phosphorylation kinase assay.
RSK1/MAPKAP-K1 α, Active
recombinant
Full length rat MAPKAP-K1α expressed in sf21 cells; phosphorylates protein
serine and threonine residues.
TAK1
human, recombinant
N-terminal histidine tag expressed in E. coli. Applications: TAK1 kinase assays.
w
Ne
M 1814
w
Ne
M 1939
w
Ne
M 2064
w
Ne
P 0365
w
Ne
R 9276
w
Ne
R 4776
w
Ne
T 3070
w
Ne
MAP Kinase
Pathway
Products Available from Sigma-RBI
MAP Kinase Activators
A 9789
Anisomycin
Isolated from Streptomyces griseolus; potent JNK agonist.
MAP Kinase Inhibitors
G 6416
GW5074
Synthetic cRaf1 kinase inhibitor. Sold for research purposes under agreement
from Glaxo Wellcome Inc. and Glaxo Group Limited.
P-215
PD 098059
Specific inhibitor of the activation of MAPKK.
R 2146
Radicicol
Antifungal macrolactone antibiotic that inhibits protein tyrosine kinase;
suppresses NIH 3T3 cell transformation by diverse oncogenes such as src,
ras and mos.
R 5010
Resveratrol
Anti-oxidant; reduces serum lipids and inhibits platelet aggregation.
w
Ne
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
17
M A P K I N A S E PAT H W AY
MAP Kinase
Pathway
MAP Kinase Inhibitors (continued)
S 8307
SB-203580
Specific inhibitor of p38 MAPK; supresses the activation of MAPKAP kinase-2;
inhibits the phosphorylation of heat shock protein (HSP) 27.
U-120
U0126
Specific inhibitor of MEK1 and MEK2; also inhibits a constitutively active,
mutant form of MEK.
MAP Kinase Substrates
M 4314
w
Ne
M 4189
w
Ne
M 5189
w
Ne
P 0240
w
Ne
MAP Kinase Substrate 1
(EGF-R [661-681])
MAP kinase 1 substrate.
MAP Kinase Substrate 4
(ERK 1/2 [172-192])
MAP kinase substrate.
MAP Kinase Substrate 3
(Tyrosine hydroxylase [24-33])
MAP kinase substrate.
p38 Regulated/Activated Protein
Kinase Peptide Substrate
Synthetic peptide.
Antibodies to MAP Kinase Adaptor Proteins
G 2791
Monoclonal Anti-Grb-2
(Clone GRB-232) Mouse purified immunoglobulin antibody; Isotype IgG3.
Applications: EL, IB, IC, IHC
Anti-Sos1
Rabbit IgG fraction of antiserum. Application: IB
w
Ne
S 2937
w
Ne
Antibodies to MAP Kinases
E 7028
w
Ne
Anti-Phospho-ERK1 [pThr202/pTyr204] Rabbit affinity isolated antibody. Applications: IB, IHC
and ERK2 [pThr185/pTyr187] (MAPK)
E 1523
Anti-ERK5
Rabbit IgG fraction of antiserum. Application: IB
E 7153
Anti-Phospho-ERK5 (BMK1)
[pThr-218/p-Tyr220]
Rabbit affinity isolated antibody. Application: IB
J 4500
Anti-Jun Kinase
Rabbit whole antiserum. Applications: IB, IS
J 4644
Anti-Phospho-JNK 1/2 (SAPK)
[pThr183/pTyr185]
Rabbit whole antiserum. Applications: IB, IS
w
Ne
w
Ne
P 1491
Anti-Phospho-p38 [pThr180/pTyr182] Rabbit whole antiserum. Applications: IB, IS
w
Ne
18
M 8159
Monoclonal Anti-MAP Kinase,
Activated (Diphosphorylated)
(Clone MAPK-YT) Mouse ascites fluid; Isotype IgG1.
Applications: IB, EL, IC, IHC, IP
A 3713
Monoclonal Anti-MAP Kinase,
Activated (Diphosphorylated),
Alkaline Phosphatase conjugate
(Clone MAPK-YT) Mouse purified immunoglobulin; Isotype IgG1.
Application: IB
F 7776
Monoclonal Anti-MAP Kinase, Activated (Clone MAPK-YT) Mouse purified immunoglobulin; Isotype IgG1.
(Diphosphorylated), FITC conjugate Application: IF
M 7802
Monoclonal Anti-MAP Kinase,
Activated (Monophosphorylated)
(Clone ERK-PT115) Mouse purified immunoglobulin; Isotype IgG1.
Applications: IB, EL, IC
M 3807
Monoclonal Anti-MAP Kinase,
nonphopshorylated (ERK1, ERK2)
(Clone ERK-NP2) Mouse purified immunoglobulin; Isotype IgG1.
Applications: IB, EL, IC
A 3960
Anti-MAP-Kinase-Agarose (ERK1, ERK2) Rabbit IgG fraction of antiserum. Application: IP
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
M A P K I N A S E PAT H W AY
M 7927
Anti-MAP Kinase (ERK-1)
Rabbit IgG fraction of antiserum. Applications: IP, IB
M 5795
Anti-MAP Kinase Kinase (MEK)
Rabbit whole antiserum. Application: IB
M 8432
Anti-p38 MAP Kinase (non-activated) (Clone P38-YNP) Mouse immunoglobulin; Isotype IgG2b.Application: IB
M 0800
Anti-p38 MAP-Kinase
Rabbit; IgG fraction of antiserum. Application: IB
M 8177
Monoclonal Anti-p38 MAP Kinase,
activated (diphosphorylated p38)
(Clone P38-TY) Mouse purified immunoglobulin; Isotype IgG2a.
Applications: IB, EL, IC
M 7681
Anti-MAP Kinase Activated
Protein Kinase-3
Sheep affinity isolated antibody. Application: IP
M 5670
Anti-MAP Kinase (ERK1, ERK2)
Rabbit whole antiserum
Application: IB
M 5437
Anti-MSK1
Rabbit IgG fraction of antiserum. Application: IB
w
Ne
MAP Kinase
Pathway
Antibodies to MAP Kinases (continued)
w
Ne
P 3237
Monoclonal Anti-phospho-PAK1 (pT212) (Clone PK-18) Mouse immunoglobulin; Isotype IgG1. Applications: IB, EL
w
Ne
R 1151
w
Ne
R 1026
Anti-c-Raf [pSer621] Phosphospecific Rabbit affinity isolated antibody. Application: IB
Antibody
Anti-c-Raf [pTyr 340/pTyr341]
Phosphospecific Antibody
Rabbit affinity isolated antibody. Application: IB
R 6525
Anti-phospho-Rsk1 (p90rsk) (pS381)
Rabbit affinity isolated antibody. Applications: IC, IP, IB
S 5183
Anti-SAPK1-β (JNK3)
Rabbit IgG fraction of antiserum. Application: IB
S 6808
Anti-SAPK3 (Erk 6)
Sheep affinity isolated antibody. Applications: IB, IP
Anti-SKK2 (300-318)
Rabbit affinity isolated antibody. Application: IB
w
Ne
w
Ne
S 6683
w
Ne
Additional MAP Kinase Antibodies Available:
C 0353
Anti-JNK, Activated-CY3
(Clone JNK-PT48)
M 7431
Monoclonal Anti-MAP Kinase 2 (Erk2)
(Clone 1B3B9)
M 3557
Monoclonal Anti-MAP Kinase,
monophosphorylated, Thr
(Clone ERK-YNP)
M 3682
Monoclonal Anti-MAP Kinase,
monophosphorylated, Tyr
(Clone ERK-NP2)
M 7556
Anti-MAP Kinase 2 (Erk2)
M 3550
Anti-MAP Kinase Activated Protein Kinase-2 (MAPKAP2)
A 4085
Anti-MAP Kinase Kinase-Agarose
(MEK)
M 7683
Anti-phospho-MAP Kinase Kinase 1&2 (MEK 1&2)
(pS218/222)
M 7808
Anti-MAP Kinase Kinase 3 (MKK3)
M 7933
Anti-phospho-MAP Kinase Kinase 3 & 6 (MKK3/MKK6)
(pS189/207)
M 0422
Anti-MAP Kinase Kinase 4 (MEK4)
M 7433
Anti-phospho-MAP Kinase Kinase 4 (MEK4, SEK1) (pT 223)
P 2979
Anti-PAK3
R 7898
Anti-B-Raf
R 7648
Anti-RAF1 (253-269)
R 5145
Anti-RSK-1 (p90)
R 5773
Anti-RAF1 (631-648)
S 5308
Anti-SKK2
R 7773
Anti-RAF1 (637-648)
S 5433
Anti-SKK5
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
19
PDK1 - PKB/AKt
Pathway
P D K 1 – P K B / A K T PAT H W AY
The PDK1–PKB/Akt axis represents one of the most actively researched cell signaling pathways. This protein kinase cascade is known to play a central role in the action of insulin, growth factors, integrins and
G protein-coupled receptors (GPCRs). It is also involved in the regulation of cell survival, metabolism
(including insulin-stimulated glucose transport and glycogen synthesis), gene expression, cell cycle entry
and protein synthesis.
All the kinases associated with this pathway lie in the protein serine/threonine kinase family and form a
single highly branching protein kinase cascade. Several of these kinases contain pleckstrin homology (PH)
domains that bind specific phosphoinositide lipids, such as phosphoinositide-3,4,5-trisphosphate (PIP3),
that are generated in the plasma membrane in response to agonist activity. As a result, kinase activation
is phosphoinositide 3-OH-kinase (PI3-kinase)-dependent.
3-Phosphoinositide-dependent protein kinase-1 (PDK1) stands at the head of this important signaling
pathway. Whether extracellular stimuli directly activate PDK1 (perhaps via the generation of plasma
membrane-localized PIP3), or whether they simply induce the translocation of PDK1 to its substrate
proteins within the plasma membrane, is not known. PDK1 activates a number of AGC-family protein
kinases (named for their homology to protein kinases A, G and C) and protein kinase B (PKB or Akt) by
phosphorylation of the T-loop Thr308. The full activation of PKB/Akt also involves the binding of PIP3
to the PH domain of PKB/Akt and the phosphorylation of an additional residue, Ser473, either by autophosphorylation, by PDK1, or by an as yet unidentified kinase called PDK2. There is a great deal of
PDK1-PKB/Akt Pathway
20
PDK1
PKB/Akt
SGK
MW (kDa)
67-69
65
SGK1: 49, SGK2α: 41, SGK2β: 47,
SGK3: 49
Domains
1 PH domain binds
PI(3,4,5)P3
1 PH domain binds
PI(3,4,5)P3 and PI(3,4)P2
Phox homology (Px) domain, catalytic
domain, glucocorticoid response element
consensus sequence activation loop,
C-terminal domain
Phosphorylation Sites
Ser25, Ser241, Ser395, Ser396, Thr308, Ser473
Ser410, Thr35, Thr513, Tyr9,
Tyr373, Tyr376
SGK1: Ser78, Thr256, Ser422;
SGK2α: Thr193, Ser279, Ser356, Ser334;
SGK2β: not known;
SGK3: Thr353, Ser419, Ser77, Ser79
Tissue Distribution
Brain, skeletal muscle
Ubiquitous; PKBγ high in brain,
lung and kidney
SGK1, SGK3: ubiquitous; SGK2α: liver,
kidney, pancreas; SGK2β: liver, kidney
Isoforms
None
PKBα, PKBβ (AKT-2),
PKBγ (AKT-3)
1, 2α, 2β, 3
Upstream Activator(s)
PI3K
PDK1, ILK
ERK5, PDK1, PI3K
Downstream Activation
PKB, p70S6K, PKC (ζ,ι,λ), PKA,
MAPKAP-K1, SGR
BAD, caspase 9, NFκB,
mTOR, GSK
B-Raf
Subcellular Localization
Cytoplasm, plasma membrane
Cytoplasm, plasma membrane
Cytoplasm, nucleus
Species
Eukaryotes
Eukaryotes
Eukaryotes
Other Names
3-Phosphoinositide Protein
Kinase-1
Protein Kinase B, RAC-PK
Serum and Glucacorticoid-Induced
Kinase
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
P D K 1 – P K B / A K T PAT H W AY
PDK1 - PKB/AKt
Pathway
Growth Factor
GRB
P
P SHC
P
P
P
P
P
P
SOS
Ras
PI-4-P
PI-4,5-P2
TEP1
PI3-Kinase
PI-3,4-P2
PI-3,4,5-P3
H2 N
PH
PDK
473
P
308
P
Akt
14-3-3
IKK-
P
p50
Bcl-X L Bad
I B
p65
Calcineurin
P
Bad
Bcl-X L
p50
p65
NF- B
Cell Survival
Cell Survival
Apoptosis
PDK1-PKB/Akt signaling promotes cell survival via two distinct pathways: 1) BAD becomes phosphorylated,
inhibiting apoptosis, or 2) IKK-α becomes activated, leading to NF-kB activation and cell survival.
GSK3
mTOR
p70S6K
GSK3α: 51, GSK3β: 47
290
70
Activation segment containing
N-terminal β-sheet domain and
C-terminal α-helix domain, dimer
16 HEAT domain,
1 PI3-kinase homology domain,
1 FKBP/rapamycin binding domain
1 Autoinhibitory domain,
1 nuclear localization sequence
on α1 and β1
GSK3α: Tyr279
GSK3β: Ser9, Tyr216
Not known
Thr229, Thr389, Ser411, Thr421, Ser424
Testis, thymus, prostate, ovary; low
expression in brain, lung and kidney
Ubiquitous
Ubiquitous
α, β
None
α1, α2, β1, β2
PKB; GSK3β: AKT1, ILK1
PKB
PKC, PDK1, mTOR
τ, NFκB, c-JUN
p70S6K, 4E-BP1
S6
Cytoplasm
Cytoplasm
Cytoplasm
Eukaryotes
Eukaryotes
Eukaryotes
Glycogen Synthase Kinase-3
Mammalian Target of Rapaymcin,
FRAP, RAFT (rat form), SEP
S6K1
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
21
PDK1 - PKB/AKt
Pathway
P D K 1 – P K B / A K T PAT H W AY
functional overlap between PKB/Akt isoforms; all phosphorylate the same RXRXXS/T motif and all are
capable of transforming a cell when rendered constitutively active by the introduction of a myristolation
signal sequence.
Thr308 and Ser473 lie within regions of PKB/Akt that are conserved throughout the AGC family kinases.
Hence, PDK1 also phosphorylates and activates several other AGC-family kinases. PDK1 is therefore a
central controller of multiple signaling pathways.
GSK3 also plays a role in the regulation of β-catenin stability and thus in gene expression. mTOR is
unusual in that it has both serine/threonine protein kinase as well as lipid kinase activities. It is a large
complex molecule that is a receptor for the immunosuppressant, rapamycin. mTOR, along with PDK1,
then plays an as yet ill-defined role in the activation of p70S6K that is important in the control of protein
synthesis, development, and growth control.
Products Available from Sigma-RBI
PDK1-PKB/Akt Enzymes
G 1663
Glycogen Synthase Kinase
Recombinant; rabbit expressed in E. coli. Application: Enzymatic assay.
S 8939
SGK1 (∆ 1-60, S422D), Active
Recombinant; human expressed in Sf9 cells. Applications: Kinase assays.
p70 S6 Kinase (T412E), Active
Recombinant; human expressed in Sf9 cells. Applications: Kinase assays.
w
Ne P 6865
w
Ne
PDK1-PKB/Akt Inhibitors
G 2911
GF 109203X
Potent GSK3 inhibitor.
R 0395
Rapamycin
Isolated from Streptomyces hygroscopicus; macrocyclic triene antibiotic
with potent immunosuppressive activity.
R-136
Ro 31-8220
p70S6K inhibitor.
G 5791
Anti-Phospho-GSK-3α/β [p Tyr279/216]
Rabbit affinity isolated antibody. Applications: IB, EL, DB
Anti-Glycogen Synthase Kinase-3β
(GSK-3β)
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-GSK-3β [pSer9]
Rabbit affinity isolated antibody. Applications: IB, EL, DB
P 3110
Anti-PDK1
Rabbit affinity isolated IgG fraction of antiserum. Application: IB
P 1601
Anti-Protein kinase Bα
Rabbit IgG fraction of antiserum. Immunogen: Application: IB
P 4112
Anti-Phospho-PKB (pSer473)
Rabbit affinity isolated IgG fraction. Application: IB
Anti-Phospho-PKB (pThr308)
Rabbit affinity isolated IgG fraction. Application: IB
Anti-S6 Kinase (p70S6K)
Rabbit affinity isolated antiserum. Application: IB
Anti-Phospho-S6 Kinase
(p70S6K) (Thr389)
Rabbit affinity isolated antibody. Application: CH
Anti-Phospho-S6 Kinase (p70S6K)
(pThr421/pSer424)
Rabbit affinity isolated antibody. Applications: CH, IP
Antibodies to PDK1-PKB/Akt Pathway
w
Ne G 7914
G 6542
w
Ne
w
Ne P 3862
w
Ne S 4047
S 6311
w
Ne
S 6436
w
Ne
22
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
Protein kinase C (PKC) is a cyclic nucleotide-independent enzyme that phosphorylates serine and threonine
residues in many target proteins. It was first identified in 1977 in bovine cerebellum by Nishizuka and
co-workers as a protein kinase that phosphorylated histone and protamine. Since then, its involvement
in many biological processes has been demonstrated, including development, memory, cell differentiation
and proliferation and carcinogenesis. Once thought to be a single protein, PKC is now known to comprise a large family of isozymes that differ in structure, cofactor requirements and function. At present,
11 isozymes have been identified, varying in tissue expression and cellular compartmentalization.
Protein Kinase C
PROTEIN KINASE C
The PKC family has been divided into three groups, based on the isozymes’ cofactor requirements: conventional (c)PKC isoforms (comprising α, βI {also known as β2}, βII {also known as β1} and γ), that
require calcium and diacylglycerol (DAG) for activation, novel (n)PKC isoforms (comprising δ, ε, η {also
known as PKC-L}, θ and µ {the mouse homolog of human PKCµ is known as PKD}) that require DAG
and atypical (a)PKC isoforms, namely ζ, ι and l (the mouse homolog of human PKCι) that require
neither calcium nor DAG. A new PKC member has recently been discovered and is referred to as PKCν.
It contains 890 amino acid residues and exhibits highest sequence similarity to PKCµ/PKD, thereby posing the possibility of a fourth subfamily of PKCs, comprising these isoforms. The PKC-related kinases
(PRKs) have also been classified as members of the PKC superfamily.
Activation of cPKCs involves translocation from the cytoplasm to binding domains at cell membranes.
Specific anchoring proteins, immobilized at particular intracellular sites, localize the kinase to its site of
action. These proteins include receptors for activated C-kinase (RACKS) and adducins. Following an
increase in intracellular calcium levels, cPKCs interact with the cell membrane in an inactive, but conformationally distinct, form. DAG facilitates penetration of these isozymes into the cell membrane. Tumorpromoting phorbol esters are used experimentally as synthetic DAG analogs. When attached, the
affinity of PKC for calcium is increased such that activation of the enzyme is achieved, depending on its
phosphorylation state. Phosphatidylserine is the membrane lipid anchor for both cPKCs and nPKCs,
although other membrane phospholipids may ultimately link extracellular signals to intracellular events
through PKC.
Each phosphorylation event induces conformational changes in the PKC molecule that result in altered
thermal stability, resistance to phosphatases and increased catalytic activity.
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
23
Protein Kinase C
PROTEIN KINASE C
Protein Kinase C
α (alpha)
β1 (beta 1)
β2 (beta 2)
γ (gamma)
δ (delta)
ε (epsilon)
Conventional
Conventional
Conventional
Conventional
Novel
Novel
MW (kDa)
76.8
76.9
76.8
78.4
77.5 (r)
83.5 (r)
Domains
Phospholipid binding domain for membrane interaction, catalytic domain, regulatory domain (with conserved regions),
variable domains (with lower homology)
Phosphorylation
Sites
Thr250, Ser657,
Thr497 (activation
loop), Thr638,
(autophosphorylation
site)
(r, m, h, rb)
Thr500
(phosphorylated
by PDK1),
Thr641 (autophosphorylation
site), Ser660 (autophosphorylation
site (r, m, h, rb)
Thr500
(activation loop),
Thr642 (autophosphorylation
site), Ser660 (autophosphorylation
site) (m, h, r)
Thr514
Thr505
(activation loop),
(activation loop),
Thr655 (autoSer643 (autophosphorylation
phosphorylation
site), Thr674 (auto- site), Ser662;
phosphorylation
Tyr332, Tyr512
site) (r, m)
(Lck, H2O2)
Thr566, Thr703,
Thr710, Ser729 (h,r) Thr565, Thr709
Ser728 (rb)
Tissue
Distribution
Ubiquitous:
CNS, heart, kidney,
liver, lung
Ubiquitous:
CNS, heart, kidney,
liver, lung
Ubiquitous:
CNS, heart, kidney,
liver, lung
CNS
Ubiquitous:
CNS, heart, kidney,
liver, lung
Ubiquitous:
CNS, heart, kidney,
liver, lung
Subcellular
Localization
Cytoplasm
Cytoplasm
Cytoplasm
Cytoplasm
Cytoplasm
Cytoplasm
Species
h, b, p, m, r, rb
h, c, b, p
h, c, b, p
h, rb, m, b
h, m, r
r, rb, m, h
Other Names
None
βII
βI
None
None
None
Upstream
Activator(s)
Ca2+, DAG, PS,
PDK-1, RACK,
PICK1
Ca2+, DAG, PS,
PDK-1, RACK
Ca2+, DAG, PS,
PDK-1, RACK
Ca2+, DAG, PS,
PDK-1, RACK
DAG, PS
DAG, PS
Downstream
Activation
MDR promoter
PKK?, PDGFβ
receptor
Not known
Not known
Not known
PIP3 (?), ATP-sensitive
K+ channels
Disease States
Multidrug resistance Diabetes,
Diabetes
(tumor resistance to Huntington’s disease
cytotoxic agents),
obesity, colon cancer
Pain
Not known
Alzheimer’s disease,
diabetes,
systemic sclerosis
Group
b: bovine
c: canine
h: human
m: mouse
p: porcine
r: rat
rb: rabbit
24
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
η (eta)
θ (theta)
µ (mu)
ζ (zeta)
ι (iota)
ν (nu)
PRK1
PRK2
Novel
Novel
Novel
Atypical
Atypical
Not named
PKC subfamily
PKC subfamily
77.6
82
115 (m)
67.7 (r)
67.2
100
104
112
Phospholipid binding domain, catalytic domain, regulatory domain (with conserved
regions), variable domains (with lower homology)
2 Zinc-dependent
phorbol-ester
domains, serine/
threonine protein
kinase catalytic
domain, 1 PH
domain
Leucine zipper-like
sequences at
N terminal,
kinase domain,
catalytic domain
Leucine zipper-like
sequences at
N terminal,
kinase domain,
catalytic domain
Thr512,
Thr655,
Ser674 (h)Thr513 (r),
Thr656 (r, m),
Tyr675 (m)
Thr538, Ser676,
Ser695 (m, h)
Not known
Thr778
Thr816
Ubiquitous:
abundant in
lung, less in
CNS, heart,
spleen
Thr744, Ser748,
Ser916 (m)
Thr410, Thr560,
Glu579 [FEY]
(r, m, h)
Thr403,
Thr574,
Thr555
Ubiquitous:
Kidney, airway
skeletal muscle, smooth muscle,
megakaryoblastic lung
cells, platelets,
CNS, heart, liver,
airway smooth
muscle, lung
Ubiquitous:
CNS, heart,
kidney, liver,
lung
Ubiquitous: Ubiquitous
CNS, heart,
airway smooth
muscle, liver,
lung
Ubiquitous: heart,
brain, placenta,
lung, skeletal
muscle, kidney,
pancreas
Ubiquitous: heart,
brain, placenta,
lung, skeletal
muscle, kidney,
pancreas
Cytoplasm
Cytoplasm
Cytoplasm
Cytoplasm
Cytoplasm
Cytoplasm
Cytoplasm
Cytoplasm
h, m, r
h, m
m, h
h, m, rb, r
h, m
Not known
h, m, r
h, m
PKC-L
None
PKD
None
PKCλ (m)
Not known
PKN
None
DAG, PS
DAG, PS
DAG, PS, PDGF
PS, PDK-1, PI3-K
PS, PI3-K
DAG
PDK-1,
Rho GTPases, S6
PDK-1, Rho GTPases,
NCK, MEKK2
Cyclin E
MDR promoter
Syk, PLCγ-1
ZIP/p62
ZIP/p62
Not known
MARCKS
eIF4E
Multidrug
resistance,
tumors
Multidrug
Not known
resistance (tumor
resistance to
cytotoxic agents),
obesity, diabetes
Tumors, diabetes
Tumors,
Not known
inflammation
Not known
Not known
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
Protein Kinase C
PROTEIN KINASE C
25
PROTEIN KINASE C
Protein Kinase C
Products Available from Sigma-RBI
Protein Kinase C Enzymes
P 2645
Protein kinase catalytic subunit
Isolated from bovine heart.
P 8289
Protein kinase catalytic subunit
Isolated from porcine heart.
P 6022
Protein kinase regulatory subunit
Isolated from bovine heart; dimer consisting of two non-covalently
linked monomers of 400 amino acid residues each.
PKC Isozymes: Human recombinant protein produced by Baculovirus-mediated expression in insect Sf9cells.
P 1782
PKCα
P 3287
PKC βII
P 1787
PKC βI
P 1164
PKCε
P 8538
PKCδ
P 0194
PKCζ
P 9542
PKCγ
P 0540
PKCη
P 7956
PKC, rat brain, lyophilized
Isolated from rat brain; mixture of isozymes.
P 0329
PKC, rat brain, solution
Isolated from rat brain; mixture of isozymes
P 1609
PKC catalytic subunit
Isolated from rat brain; does not require Ca2+ or phosphatidylserine
for its activity; prepared by tryptic digestion of PKC.
PKCα isozyme
Isolated from rat brain.
w
Ne
P 8311
Protein Kinase C Activators
B 7431
Bryostatin 1
PKC activator.
Ingenol
PKCδ and ε activator; diterpene related to phorbol.
L 0521
Lipoxin A4
Potent human PKC activator; inhibits cytotoxicity of natural
killer cells.
M 5518
Mezerein
Phorbol ester analog from the plant Daphne mezereum;
PKC activator; potent second stage tumor promotor.
O 1008
Oleic acid
PKC activator in hepatocytes.
P 9143
Phorbol 12,13-diacetate
Less potent, but more water soluble than phorbol 12,13-dibutyrate.
P 1269
Phorbol 12,13-dibutyrate
PKC activator; less hydrophobic than phorbol myristate acetate.
P 9018
Phorbol 12,13-didecanoate
PKC activator; weaker than phorbol myristate acetate as a tumor
promoting agent.
P 8014
4α-Phorbol 12,13-didecanoate
Phorbol that is not biologically active; can be used as a negative
control.
P 8139
Phorbol 12-myristate 13-acetate
PKC activator in vivo and in vitro; potent tumor promotor in mouse
skin.
P-148
4α-Phorbol 12-myristate 13-acetate
Negative control for phorbol ester activation of PKC.
P 2303
Protein kinase C fragment 530-558
Part of the catalytic domain of PKC; potent activator
of the enzyme.
T 7068
Thymeleatoxin
Selective PKCα, β1 and γ activator.
w
NeI 3381
w
Ne
Protein Kinase C Inhibitors
The following products are PKC inhibitors.
B 6292
26
Bisindolylmaleimide I
I 7016
H-7 DiHCl
R 5648
I 6891
H-7
B 1427
H-89 HCl
I 1392
HA-100 HCl
N-161
NPC-15437 DiHCl
Rottlerin
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
PROTEIN KINASE C
C 6303
Calphostin C
Isolated from Cladosporium cladosporioides; highly specific PKC
inhibitor; an inhibitor of the regulatory domain of PKC.
C 2932
Chelerythrine chloride
Benzophenanthridine alkaloid that is a potent and specific PKC
inhibitor, interacts with the catalytic domain of PKC; may also
effect translocation of PKC from cytoplasm to plasma membrane.
D 2064
Dequalinium analog, C14 linker
Selective, photo-inducible PKCα inhibitor; antitumor agent.
D 7033
Dihydrosphingosine, DL-threo
PKC inhibitor; biosynthetic precursor of sphingosine. Mixture of
erythro and threo isomers.
D 6908
Dihydrosphingosine, DL-erythro-
D-Isomer
G 1274
HA-1004 HCl
Structural analogue of H7 that is a poor inhibitor of PKC; serves as
an excellent negative control for H-7.
H 6772
Hexadecylphosphocholine
PKC and phosphatidylcholine biosynthesis inhibitor.
H 5257
Hispidin
Potent PKCβ inhibitor; cytotoxic for cancer cells.
H 9252
Hypericin
Isolated from Hypericum perforatum (St. John’s Wort); potent PKC
inhibitor.
I 2764
ML-7
Inhibits PKC at micromolar concentrations.
P 4509
Palmitoyl-DL-carnitine chloride
Specific PKC inhibitor; intermediate in mitochondrial fatty acid
oxidation.
P 8462
PKC fragment 19-36
Specific PKC inhibitor; inhibits both autophosphorylation and protein substrate phosphorylation.
P 2239
PKCζ pseudosubstrate
Selective PKCζ inhibitor.
PKCη pseudosubstrate
Selective PKCη inhibitor.
PKCθ pseudosubstrate
Selective PKCθ inhibitor.
PKCζ pseudosubstrate,
myristoylated
Cell-permeable PKCζ inhibitor.
PKCη pseudosubstrate,
myristoylated
Cell-permeable PKCη inhibitor.
PKCθ pseudosubstrate,
myristoylated
Cell-permeable PKCθ inhibitor.
R-136
Ro 31-8220
PKC inhibitor; GRK-5 (G protein-coupled receptor kinase) inhibitor.
R-137
Ro 32-0432
Selective cell-permeable PKC inhibitor with greater selectivity for
PKCα and β1 vs. ε; GRK-5 (G protein-coupled receptor kinase)
inhibitor.
S 7049
Sphingosine, D-
Selective PKC inhibitor; precursor of ceramide, natural isomer of
sphingosine.
S 4400
Staurosporine
Isolated from Streptomyces sp.; some selectivity as a PKC inhibitor.
T 5648
Tamoxifen
PKC inhibitor; induces apoptosis in human malignant glioma cell lines.
T 9262
Tamoxifen citrate
Water soluble form of T 5648.
T 3126
Tocopherol Acid succinate, (+)-α-
Semisynthetic from natural α-tocopherol; antioxidant; PKC
inhibitor; suppresses c-myc and c-H-ras oncogene expression.
w
Ne
Protein Kinase C
Protein Kinase C Inhibitors (continued)
is the biosynthetic precursor of sphingosine; negative
control for inhibition of PKC.
w
Ne
w
Ne
P 2114
w
Ne
P 1989
w
Ne
P 1614
w
Ne
P 1864
w
Ne
P 1739
w
Ne
w
Ne
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
27
PROTEIN KINASE C
Protein Kinase C
Protein Kinase C Substrates
The following products are PKC substrates:
H 4524
Histone type III-SS from calf thymus
L 9905
Lys-Arg-Thr-Leu-Arg-Arg trifluoroacetate
M 6913
Myelin basic protein fragment 4-14
N 7279
Neurogranin fragment 28-43
P 2186
pGlu-Lys-Arg-Pro-Ser-Gln-Arg-Ser-Lys-Tyr-Leu P 5307
P 1835
(Ser25)-PKC fragment 19-31
P 6114
PKCε substrate
Phospholipid/phorbol ester-dependent substrate for PKCε and ζ.
PKCζ substrate, biotinylated
Biotinylated form of the PKCζ substrate.
PKCη substrate, biotinylated
Biotinylated form of the PKCη substrate.
PKCθ substrate, biotinylated
Biotinylated form of the PKCθ substrate.
Pro-Leu-Ser-Arg-Thr-Leu-Ser-Val-Ala-Ala-Lys-Lys
w
Ne
P 2364
w
Ne
P 2614
w
Ne
P 2489
w
Ne
Antibodies to Protein Kinase C
P 5704
Monoclonal Anti-PKC
(Clone MC5) Mouse ascites fluid; isotype IgG2a.
Applications: IHC, IP, IB
P 4334
Anti-PKCα
Rabbit whole antiserum. Application: IB
P 3078
Anti-PKCβ1
Rabbit whole antiserum. Applications: DB, IB
P 6959
Monoclonal Anti-PKCβ1
(Clone PK-B13) Mouse ascites fluid; isotype IgG2b.
Applications: IB, DB
P 3203
Anti-PKCβ2
Rabbit affinity isolated antibody. Applications: DB, IB, EL
P 2584
Monoclonal Anti-PKCβ2
(Clone PK-B26) Mouse ascites fluid; isotype IgG1. Applications: IB, EL
P 3328
Anti-PKCγ
Rabbit affinity isolated antibody. Applications: DB, IB
P 8333
Anti-PKCδ
Rabbit whole antiserum. Applications: DB, IB
P 8458
Anti-PKCε
Rabbit whole antiserum. Applications: DB, IB
P 0713
Anti-PKCζ
Rabbit whole antiserum. Application: IB
P 6111
Anti-PLCγ−1 [p Tyr783]
Rabbit affinity isolated antibody. Application: IB
P 8104
Anti-PLCγ−1
Rabbit affinity isolated antibody. Applications: IP, CH
P 3987
Anti-Protein Kinase D (PKD)
Rabbit IgG fraction of antiserum. Applications: IB, IP
w
Ne
w
Ne
28
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
Protein tyrosine kinases (PTKs) are enzymes that catalyze the phosphorylation of tyrosine residues. There
are two main classes of PTKs: receptor PTKs and cellular, or non-receptor, PTKs. These enzymes are
involved in cellular signaling pathways and regulate key cell functions such as proliferation, differentiation, anti-apoptotic signaling and neurite outgrowth. Unregulated activation of these enzymes, through
mechanisms such as point mutations or overexpression, can lead to various forms of cancer as well as
benign proliferative conditions. Indeed, more than 70% of the known oncogenes and proto-oncogenes
involved in cancer code for PTKs. The importance of PTKs in health and disease is further underscored
by the existence of aberrations in PTK signaling occurring in inflammatory diseases and diabetes.
Protein
Tyrosine Kinases
PROTEIN TYROSINE KINASES
Receptor PTKs possess an extracellular ligand binding domain, a transmembrane domain and an intracellular catalytic domain. The transmembrane domain anchors the receptor in the plasma membrane,
while the extracellular domains bind growth factors. Characteristically, the extracellular domains are comprised of one or more identifiable structural motifs, including cysteine-rich regions, fibronectin III-like
domains, immunoglobulin-like domains, EGF-like domains, cadherin-like domains, kringle-like domains,
factor VIII-like domains, glycine-rich regions, leucine-rich regions, acidic regions and discoidin-like domains.
The intracellular kinase domains of receptor PTKs can be divided into two classes: those containing a
stretch of amino acids separating the kinase domain and those in which the kinase domain is continuous.
Activation of the kinase is achieved by ligand binding to the extracellular domain, which induces dimerization of the receptors. Receptors thus activated are able to autophosphorylate tyrosine residues outside
the catalytic domain via cross-phosphorylation. The results of this auto-phosphorylation are stabilization
of the active receptor conformation and the creation of phosphotyrosine docking sites for proteins which transduce signals
Integrin
within the cell. Signaling proteins which bind to the intracellular
α β
domain of receptor tyrosine kinases in a phosphotyrosinedependent manner include RasGAP, PI3-kinase, phospholipase
Talin
Cav-1
Cγ, phosphotyrosine phosphatase, SHP and adaptor proteins
Calpain
Fyn or
SOS
Yes
such as Shc, Grb2 and Crk.
Shc
Src
Grb2
FAK
Ras
Cas
Raf-1
Crk-C3G
MEK
Rap-1
ERK
B-Raf
Fyn, Yes, Src and FAK involvement
in integrin signaling.
In contrast to receptor PTKs, cellular PTKs are located in the
cytoplasm or nucleus or are anchored to the inner leaflet of the
plasma membrane. They are grouped into eight families: Src, JAK,
Abl, FAK, Fps, Csk, Syk and Btk. Each family consists of several
members. With the exception of homologous kinase domains
(Src Homology 1, or SH1 domains), and some protein-protein
interaction domains (SH2 and SH3 domains), they have little in
common, structurally. Of those cellular PTKs whose functions
are known, many, such as Src, are involved in cell growth. In
contrast, Fps PTKs are involved in differentiation, Abl PTKs are
involved in growth inhibition, and FAK activity is associated with
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
29
Protein
Tyrosine Kinases
PROTEIN TYROSINE KINASES
30
cell adhesion. Some members of the cytokine receptor pathway interact with JAKs, which phosphorylate the transcription factors, STATs. Still other PTKs activate pathways whose components and functions
remain to be determined.
Receptor Tyrosine Kinases
PDGFR
IGFR
FGFR
IR
Family
RTK
RTK
RTK
RTK
MW (kDa)
PDGFR-α 170, PDGFR-β 190 Type I 130; Type II 250
130-150
α-subunit: 135,
β-subunit: 95
Domains
Transmembrane
domain
Cytoplasmic tyrosine
kinase domain,
transmembrane
domain, 2 fibronectin
Type III-like domains;
Type I contains 2 α and
2 β subunits
Cytoplasmic kinase domain,
transmembrane domain;
3 Ig-like domains: D1, D2,
D3-D3 has alternate splice
variants IIIb, IIIc
Tetramer of 2α
and 2β subunits;
β-chain contains
kinase domain; 2
fibronectin type-IIIlike domains
Phosphorylation
Sites
Tyr579, Tyr740,
Tyr857, Tyr1009 (β)
Tyr590, Tyr591,
Tyr786, Tyr1131,
Tyr1135, Tyr1136,
Tyr1141, Tyr1150,
Tyr1151, Tyr1232,
Tyr1246, Ser1280,
Ser1283
FGFR1 -Tyr463/730,
Tyr583, Tyr585,
Tyr653, Tyr654,
Tyr730, Tyr766
Tyr1146, Tyr1150,
Tyr1151, Ser1275,
Ser1309
Tissue Distribution
Mesenchymal cells
smooth muscle cells
lungs, CNS, peripheral
nervous system
Ubiquitous
Most types of cells
including parathyroid
cells, kidney carcinoma,
endothelial; mesenchymal
skin, brain, skeletal muscle
Present in most
tissues
Isoforms
PDGFR-α, PDGFR-β
IGFR-I, IGFR-II
FGFR -1, -2, -3, -4
None
Subcellular Localization Plasma membrane
Plasma membrane
Plasma membrane
Plasma membrane
Species
Human, mouse,
Drosophila, rat
Human, mouse, Drosophila, Human, mouse, rat, chick,
hamster, chicken
Xenopus
Other Names
Platelet-derived growth
factor receptor
Insulin-like growth
factor receptor; Type II300 kDa mannose-6phosphate receptor
Fibroblast growth factor
receptor, FGFR1/flg, c-FGR,
FGFR2/bek
Insulin receptor
Upstream Activator(s)
PDGF
IGF-I, IGF-II
Acidic and basic FGF
Insulin
Downstream
Activation
Grb2 (Ras), Src, PI3K
Insulin receptor substrate
1 and 2, Shc (leads to
recruitment of Grb2 and
activation of PI3K and
MAPK pathways)
PLCγ, Shc adaptor protein,
Raf-1
Insulin Receptor
Substrates (IRS),
Shc, proteintyrosine
phosphatase-1B
(PTP1B)
Disease States
Chronic myelemonocytic
leukemia (CMML), actute
myelogenous leukemia
Tumors; Type II: BeckwithWiedemann syndrome
Pfeiffer syndrome
Diabetes mellitus,
Leprechaunism
(Donohue syndrome)
Mammals, fish,
C. elegans, Xenopus
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
PROTEIN TYROSINE KINASES
EGFR
HER-2/neu/ErbB2
HER-3/ErbB3
HER-4/ErbB4
Family
RTK
RTK
RTK
RTK
MW (kDa)
180-190
185
160
180
Domains
Extracellular ligand-binding
domain, hydrophobic
transmembrane region,
intracellular tyrosine
kinase domain
Extracellular ligand-binding
domain, hydrophobic
transmembrane region,
intracellular tyrosine
kinase domain
Extracellular ligand-binding
domain, hydrophobic
transmembrane region,
cytoplasmic domain,
intracellular tyrosine
kinase domain
Extracellular ligand-binding
domain, hydrophobic
transmembrane region,
cytoplasmic domain,
intracellular tyrosine
kinase domain
Phosphorylation Sites
Tyr703, Tyr789, Tyr845,
Tyr882, Tyr899, Tyr958,
Autophosphorylates,
Tyr891, Tyr920, Tyr992,
Tyr1028, Tyr1143, Tyr1226, exact sites not known
Tyr1068, Tyr1086, Tyr1101, Tyr1227, Tyr1253
Tyr1114, Tyr1148, Tyr1173,
Thr654, Thr669, Ser1046,
Ser1047
Tyr1162, Tyr1188, Tyr1258,
Tyr1284 (precursor)
Tissue Distribution
Endocytic vesicles, vulvar
precursor cells, cornea,
epidermis, dermis, liver,
pancreas, nerve, amnion
adrenal medulla, colon,
mammary, bladder
Mammary gland, cortical
neurons, CNS, astrocytes,
Schwann cells, skeletal
muscle cells, gastric cells,
salivary cells
Epithelial tissue, skeletal
muscle and brain
Expressed in highest levels
in brain, heart, kidney,
skeletal muscle, parathyroid, cerebellum,
pituitary, spleen, testis and
breast. Lower levels in
thymus, lung, salivary
gland and pancreas. Both
isoforms are expressed in
cerebellum, but only the
JM-β isoform is expressed
in heart
Isoforms
None
None
None
HER4 JM-α, HER4 JM-β
Subcellular Localization Plasma membrane
Plasma membrane,
nucleus
Plasma membrane,
secreted
Plasma membrane
Species
Human, mouse, rat,
C. elegans, Drosophila
Human, mouse, rat,
C. elegans, Drosophila
Human, mouse, rat
Human, mouse, rat
Other Names
ErbB1
Neu (rat), c-ErbB2
epidermal growth
factor receptor
Human epidermal growth c-ErBβ-4, p180erbB4
factor receptor 3;
epidermal growth
p180erbB3, c-ErBβ-3
factor receptor
Upstream Activator(s)
EGF, TGFα,
amphiregulin
GP30 (potential), NRG
Heregulins (neurogulins
α/β), NTAK, EFG/TGFα,
β-cellulin
EGF, heregulin
(neuregulin), NRG-2,
NRG-3, Heparin-binding
EGF-like growth factor,
β-cellulin, NTAK
Downstream
Activation
Forms heterodimer with
HER-2, HER-3, HER-4;
Ras, Jnk, FAS,
PI3K, cSrc, Raf-I, PKC
Forms heterodimer with
EGFR, HER-3, HER-4;
PI3K, Src, Ras, Shc,
Grb2, Crk
Forms heterodimer with
EGFR, HER2; PI3K, Src,
ras-GAP, PLCγ, Shc,
Grb-2, Grb-7, Crk
Forms heterodimer with
EGFR, HER2, HER3; PI3K,
Src, ras-GAP, PLCγ, Shc,
Grb-2, Grb-7, Crk, c-Cbl,
c-Abl, Shp2
Disease States
Cancer of the head and
neck, lung, pancreas,
bladder and breast
Cancer of colon, ovary,
bladder and breast
Mammary tumors,
hepatocellular carcinoma
Breast carcinoma (unclear)
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
Protein
Tyrosine Kinases
Receptor Tyrosine Kinases
31
PROTEIN TYROSINE KINASES
Protein
Tyrosine Kinases
Non-Receptor Tyrosine Kinases
c-Src (pp60)
c-Yes
Fyn
Lyn
Family
Non-receptor TK
Non-receptor TK
Non-receptor TK
Non-receptor TK Src family
MW (kDa)
60
62
59
A isoform: 58 (h,m), 59 (r)
Domains
SH1 catalytic domain,
SH2 domain,
SH3 domain
SH1 catalytic domain,
SH2 domain,
SH3 domain
SH1 catalytic domain,
SH2 domain, SH3 domain,
myristylated N-terminal
glycine residue
N-terminal myristoylation
domain, a unique domain,
SH2 and SH3 domains, a
protein kinase domain,
and a C-terminal
regulatory domain
Phosphorylation Sites
Tyr215, Tyr416, Try418,
Tyr424 (m), Tyr537,
Tyr430, Tyr527, Tyr530,
Tyr700, Tyr731, Tyr774
Tyr1292, Tyr1325, Tyr1387
Tyr308, Tyr1472, Tyr531,
Ser473
Tyr416 (autophophorylation site), Tyr527
Tissue Distribution
Many tissues including
epithelial cells, platelets,
osteoclasts, neurons
Many tissues including
liver, lung, placenta,
platelets, keratinocytes,
brain, epithelial cells
in kidney
Fibroblasts,
endothelial cells,
lymphocytes, monocytes,
T-cells, platelets, neurons
B-cells, platelets
Isoforms
c-src1 (pp60); c-src2
None
Fyn T, Fyn B
Lyn A, Lyn B
Cytoplasm
Cytoplasm
Cytoplasmic membrane
and cytoplasm (endocytic
vesicles and coated pits)
Subcellular Localization Cytoplasm,
plasma membrane
32
Species
Avian (v-Src), human (s-Src), Human, yeast,
mouse, Xenopus, rat
avian (v-Yes),
dog, mouse, Xenopus
Rat, mouse, human,
chicken, Xenopus
Human, mouse,
rat, chicken
Other Names
Rous sarcoma virus,
Oncogene Src,
Protooncogene Src,
Src Oncogene,
oncogene protein pp60
Oncogene Yes1,
pp62-Yes
Fyn, Tyrosine Kinase
Prooncogene, pp59,
Syn
None
Upstream Activator(s)
Csk, Cdk, PDGF-β
Csk, PDGF-β,
CD36 (platelets)
Csk, PDGF-β, CD45,
CD36 (platelets),
Bcr (B-lymphocytes),
Tcr (T-lymphocytes)
Bcr (Iga, Igb), CD45, Csk
Downstream
Activation
EGFR, Shc, dynamin,
clathrin, Raf-1, JAK1,
STAT1, STAT3, STAT5,
G-protein-linked receptor
kinase 2, caveolin-1
Pyk2
PI3K, p120/130, Cbl,
Pyk2
Btk, Syk, Cbl, p85 subunit
of PI3K
Disease States
Embryonic development, Colon carcinomas,
colon cancer, osteoporosis hematopoietic disorders
Neurological diseases impaired spatial learning
Glomerulonephritis, IgM
hyperglobulinemia
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
PROTEIN TYROSINE KINASES
Lyk
Hyk
Btk
Csk
Bcr-Abl
Family
Non-receptor TK
BTK family
Non-receptor TK
Non-receptor TK
Non-receptor TK
Non-receptor TK
MW (kDa)
72
126 (precursor)
76 (h, m)
50
210, 190, 230
Domains
SH2 and
SH3 domains,
PH domain,
kinase domain
2 Ig-like
C2-type domains,
3 fibronectin type
III-like domains,
3 EGF-like domains,
protein kinase
domain
C-terminal
PTK domain,
PH domain, a
proline-rich Tec
homology domain,
SH2 and SH3
domains
SH3 and SH2
domains, proline rich
region (PPPLPERTP) in
the non-catalytic
C-terminal domain
N-terminal serine/
threonine kinase
domain, PH domain,
SH2 domain, SH3
domain
Phosphorylation
Sites
Tyr512 (autophosphorylation), Tyr317
Tyr992 (autophosphorylation in
precursor),Tyr1100,
Tyr814, Tyr1106
Tyr223, Tyr551
Tyr416 (autoTyr177, Tyr328, Ser354,
phosphorylation site), Tyr 360
Ser364
Tissue Distribution T-cells, natural
killer cells
Endothelial cells
B-cells, platelets
with higher levels
in placenta and lung,
and lower levels
in brain and kidney
Ubiquitous
Hematopoietic cells
Isoforms
None
None
None
None
p190, p210, p230
Subcellular
Localization
Plasma membrane,
cytoplasm
Plasma membrane
Cytoplasm
Cytoplasm
Cytoplasm
Species
Human, bovine,
mouse
Human, mouse,
bovine
Human, mouse,
chicken
Yeast, human,
mouse, rat, chicken
Human, mouse, rat
Other Names
T-cell specific kinase,
ITK, TSK, EMT
TIE-2, TEK
Bruton’s TK,
ATK, BPK,
Agammaglobulinaemia TK
c-Src tyrosine kinase, Breakpoint cluster
CYL
region, Abelson
oncogene
Upstream
Activator(s)
TCR, CD28,
PtdIns(3)P,
PtdIns(3,4)P2,
PtdIns(3,4,5)P3
Angiopoietin 1,4
Lyn,
PtdIns-3,4,5-P3,
Bcr
PKA
Chromosomal translocation creates oncogenic fusion protein
which is constitutively
active
Downstream
Activation
Socs-1
Grb2, Grb7, Grb14,
Shp2, Dok-R,
p85 subunit of
PI3-K
Src kinase, Lyn, Fyn,
Lck
PI3K, STAT1, STAT5,
STAT6, Ras, PKC, IL-3,
paxillin, vinculin, actin,
Fak, c-Abl, Src, Bcl-2
Disease States
Immunodeficiency
Dominantly
inherited venous
malformations
(VMCM1)
PLC-γ2, STAT5A
X-linked
Not known
agammaglobulinemia
Protein
Tyrosine Kinases
Non-Receptor Tyrosine Kinases
Chronic myelogenous
leukemia (CML), acute
lymphocytic leukemia,
Philadelphia chromosome (Ph1)-positive
acute lymphocytic
leukemia (ALL)
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
33
PROTEIN TYROSINE KINASES
Protein
Tyrosine Kinases
Non-Receptor Tyrosine Kinases
34
SYK
ZAP70
FES
FER/TYK3
FAK
Family
Non-receptor TK
Non-receptor TK
Non-receptor TK
Non-receptor TK
Non-receptor TK
MW (kDa)
72
70
93
51, 94
FAK1: 119
Domains
C-terminal SH2,
N-terminal SH2,
kinase domain
SH2 domain,
SH1 domain,
kinase domain
SH2 domain,
kinase domain,
unique N-terminal
domain with 2
coiled coil-forming
motifs
Tyrosine protein
kinase domain;
lacks the transmembrane and
extracellular
domains. Fer has
SH2 and kinase
domain, iFer lacks
kinase domain
Proline-rich region;
T. FAK paxillin binding
sequence contains talin
binding sequence,
N-terminal domain
binds integrin
β subunit.
Phosphorylation
Sites
Tyr130,
Tyr317,
Tyr345,
Tyr519,
Tyr623,
Tyr126, Tyr292,
Tyr315, Tyr319,
Tyr492, Tyr493
Tyr713, Tyr811
Tyr421, Tyr466,
Tyr482, Tyr714
(autophosphorylation
site)
Tyr397 (autophosphorylation site)
Tyr407, Tyr576, Tyr577
Tyr861, Tyr925, Ser722,
Ser840, Ser843, Ser910
Tissue Distribution Spleen, thymus,
hematopoietic cells
(B-cells), breast
T-cells,
natural killer cells
Myeloid hematoUbiquitous
poietic cells, the
vascular endothelium
All organs, lymphoid
tissues, levels high
in the brain
Isoforms
SYKA, SYKB
None
None
Fer, iFer
FAK1, FAK2, FAK3,
FAK4
Subcellular
Localization
Cytoplasmic
membrane,
cytoplasm
Cytoplasmic
membrane,
cytoplasm
Cytoplasm
Cytoplasm,
nucleus
Cytoplasm
Species
Human, mouse,
rat, pig
Human, mouse
Birds, human,
mouse, cat, rat
Human, mouse
Human, mouse, rat,
chicken, frog
Other Names
Spleen tyrosine
kinase
70 kDa
ζ-associated
protein, SRK
(Syk-related
tyrosine kinase)
Fps/Fes; FeSV
Protein tyrosine
(feline sarcoma virus), kinase 3;
c-FES
FPS/FES-related
tyrosine kinase
Focal adhesion kinase,
FADK
Upstream
Activator(s)
Lyn
TCR-z, Lck
IL-3, Erythropoietin
PDGF
Integrin β1, β2, β3
cytoplasmic tails
Downstream
Activation
PLC-γ2, Shc
Not known
Bcr, Ras-GAP, Shc
β-catenin
Cas, Shc, Grb2,
PI3-kinase
Disease States
Tumor suppresor in
breast carcinomas
Human immunodeficiency, selective
T-type defect
Diabetes mellitus,
malignancies
Cancer
Cancer
Tyr290,
Tyr341,
Tyr358,
Tyr525,
Tyr624
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
PROTEIN TYROSINE KINASES
JAK1
JAK2
JAK3
TYK2
Family
Non-receptor TK
Non-receptor TK
Non-receptor TK
Non-receptor TK JAK family
MW (kDa)
132
131
125
134
Domains
7 JAK homology
(JH) domains,
JH1 kinase domain,
JH2 pseudokinase domain
7 JAK homology
(JH) domains,
JH1 kinase domain,
JH2 pseudokinase domain
7 JAK homology
(JH) domains,
JH1 kinase domain,
JH2 pseudokinase domain
7 JAK homology
(JH) domains,
JH1 kinase domain,
JH2 pseudokinase domain
Phosphorylation Sites
Tyr1022, Tyr1023, Tyr1033
Tyr1007, Tyr1008
Tyr1033, Tyr980, Tyr981
Tyr1054, Tyr1055
Tissue Distribution
Ubiquitous
Ubiquitous
Primarily hemopoietic
tissues, epithelial cells
Ubiquitous
Isoforms
None
None
JAK3M, JAK3B, JAK3S
None
Subcellular Localization Cytoplasm
Cytoplasm
Cytoplasm
Cytoplasm, nucleus
Species
Vertebrate, mammalian,
rat, mouse, metazoa,
plants, fungi, Drosophila
Vertebrate, mammalian,
rat, mouse, metazoa,
plants, fungi, Drosophila
Vertebrate, mammalian,
rat, mouse, metazoa,
plants, fungi, Drosophila
Human, mouse
Other Names
Janus Kinase 1
Janus Kinase 2
Janus Kinase 3,
leukocyte Janus kinase,
L-JAK
Tyrosine kinase-2
Upstream Activator(s)
IFNα, IFNγ, IL-2,
IL-4, IL-9, IL-13, IL-15,
IL-3, IL-6, IL-11, CT-1,
CNTF, LIF, OSM,
prolactin
IFNγ, EPO, IL-3, IL-5,
GM-CSF, IL-6, IL-11,
CT-1, CNTF, OSM
IL-2, IL-4, IL-7, IL-9, IL-15
IFNα, IL-12, IL-13, IL-6,
IL-11, OSM, CNTF, LIF, CT-1
Downstream
Activation
STAT, cytokine receptor
STAT, cytokine receptor
STAT, cytokine receptor,
IRS1, IRS2, PI3K
STAT, cytokine receptor
(IFNAR1)
Disease States
Tumorigenesis, leukemias, Diseases of abnormal
myocardial ischemia
erythropoiesis, myeloproliferative disorders,
immunosuppressive
diseases
Protein
Tyrosine Kinases
Non-Receptor Tyrosine Kinases
Severe combined
Immune diseases
immunodeficiency disorder
(SCID), lymphoproliferative
disorder
Products Available from Sigma-RBI
Protein Tyrosine Kinase Kits
PTK-101
Protein tyrosine kinase (PTK) assay kit
L 3539
Lck (P56Lck), active
Enables the detection, measurement, and characterization of protein
tyrosine kinase (PTK); based on an ELISA assay format using the universal
PTK substrate, a phosphotyrosine specific monoclonal antibody (clone PT-66)
conjugated to peroxidase (HRP) and a positive tyrosine kinase control.
Protein Tyrosine Kinase Enzymes
w
Ne
Human, recombinant, expressed in Sf21 insect cells; tyrosine kinase
which activates T-lymphocytes.
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
35
Protein
Tyrosine Kinases
PROTEIN TYROSINE KINASES
Protein Tyrosine Kinase Inhibitors
C 2932
Chelerythrine chloride
Inhibits tyrosine protein kinase when used at micromolar concentrations.
D 7802
Daidzein
Less active analog of the tyrosine kinase inhibitor, genistein; phytoestrogen
recently suggested to play a role in preventing hormone-induced cancers.
D-210
4,5-Dianilinophthalimide
Protein tyrosine kinase inhibitor with selectivity for the epidermal growth
factor (EGF) receptor.
D 2667
2,5-Dihydroxycinnamic acid methyl ester A stable analog of erbstatin; EGF receptor-associated kinase inhibitor.
E 7881
Emodin
Tyrosine kinase inhibitor; inhibitor of NFκB activation and adhesion
molecule expression.
G 3381
Geldanamycin
A potent antitumor antibiotic; inhibitor of proto-oncogenic protein kinases,
such as erbβ2, EGF receptor tyrosine kinases and non-receptor tyrosine
kinases such as v-src; potent nuclear hormone receptor family inhibitor.
G 6649
Genistein
Tyrosine protein kinase inhibitor.
G 0897
Genistin
Inactive analog of genistein; useful as a negative control for genistein.
H 6649
Herbimycin A
Cell-permeable tyrosine kinase inhibitor; inhibits platelet-derived growth
factor-induced phospholipase D activation.
L 2400
Lavendustin A
Cell-permeable tyrosine kinase inhibitor with little effect on protein
kinase A or C.
L 5025
Leflunomide
Immunosuppressive; inhibits T- and B-cell proliferation. Activity is attributed mainly to its metabolite, a malononitrile derivative, which is
believed to inhibit several protein tyrosine kinases.
P 0453
Piceatannol
Plant metabolite possessing antileukemic activity; inhibits protein tyrosine
kinases Syk, p40 and p56.
The following products are Epidermal growth factor (EGF) receptor tyrosine kinase inhibitors:
T 7165
Tyrphostin 23
T 7290
Tyrphostin 25
T 7540
Tyrphostin 47
T 7665
Tyrphostin 51
T 7790
Tyrphostin 63
T 4182
Tyrphostin AG 1478
T 9177
Tyrphostin AG 126
Tyrosine kinase inhibitor that blocks production of tumor necrosis factor-α
(TNF-α) and nitric oxide in macrophages.
T 2067
Tyrphostin AG 879
Nerve growth factor receptor (TrkA) tyrosine kinase inhibitor; inhibits
140 trk protooncogene and HER-2.
T 4057
Tyrphostin AG 1296
Selective platelet-derived growth factor (PDGF) receptor tyrosine kinase
inhibitor.
T 5317
Tyrphostin AG 1433
PDGFβ receptor tyrosine kinase inhibitor; VEGF Kinase inhibitor.
T 3434
Tyrphostin AG 490
JAK-2 protein tyrosine kinase inhibitor.
T 4192
Tyrphostin SU 1498
Potent and selective VEGF receptor tyrosine kinase inhibitor, Flk-1; very
weak PDGFR-kinase, EGFR-kinase and HER-2 kinase inhibitor.
Tyrphostin 1
Acts as a negative control against Tyrphostin 23, 25, 46, 47 and 51.
w
Ne
T 7040
36
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
PROTEIN TYROSINE KINASES
A 7433
Arg-Arg-Leu-Ile-Glu-Asp-Ala-Glu-TyrAla-Ala-Arg-Gly
C-276
Lys-Lys-Lys-Lys-Glu-Glu-Ile-Tyr-Phe-Phe-Phe C-Terminal Src kinase (Csk) substrate.
w
Ne T 7195
w
Ne T 7320
w
Ne T 7445
w
Ne
Insulin receptor tyrosine kinase substrate.
Tyrosine kinase peptide 1
Substrate for various tyrosine kinases.
Tyrosine kinase peptide 2
Substrate for various tyrosine kinases.
Tyrosine kinase peptide 3
Substrate for various tyrosine kinases.
Protein
Tyrosine Kinases
Protein Tyrosine Kinase Substrates
Antibodies to Protein Tyrosine Kinase Adapter Proteins
C 0354
Anti-p130(CAS)
Rabbit IgG fraction of antiserum. Application: IB
Anti-c-Cbl
Rabbit IgG fraction of antiserium. Application: IB
C 0853
Anti-Crk-II
Rabbit IgG fraction of antiserium. Application: IB
C 0978
Anti-Crk-L
Rabbit IgG fraction of antiserium. Applications: IB, IP, IF
G 2791
Anti-Grb2
(Clone GRB-232) Mouse purified immunoglobulin; isotype IgG3.
Applications: IB, IP, IHC, IC,EL
w
Ne C 9603
w
Ne
Antibodies to Protein Tyrosine Kinases
F 2918
Anti-Focal Adhesion Kinase (P125 FAK) Rabbit IgG fraction of antiserum. Applications: IB, IP, IC, IF
F 7926
Anti-Phospho-FAK [p-Tyr397]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-FAK [p-Tyr407]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-FAK [p-Tyr576]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-FAK [p-Tyr577]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-FAK [p-Ser722]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-FAK [p-Tyr861]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-FAK [p-Ser910]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-FAK [p-Tyr925]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-Pyk2 [p-Tyr402]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-Pyk2 [p-Tyr579]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-Pyk2 [p-Tyr580]
Rabbit affinity isolated antibody. Application: IB
w
Ne F 8051
w
Ne
F 8801
w
Ne F 8926
w
Ne F 9051
w
Ne F 9176
w
Ne F 9301
w
Ne F 9426
w
Ne P 6614
w
Ne P 7714
w
Ne P 6739
w
Ne P 6989
w
Ne P 6864
w
Ne J 4019
J 3251
w
Ne J 4269
J 3376
w
Ne J 3877
w
Ne J 3252
w
Ne
P 3902
w
Ne S 2440
w
Ne
Anti-Phospho-Pyk2 [p-Tyr579/p-Tyr580] Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-Pyk2 [p-Tyr881]
Rabbit affinity isolated antibody. Application: IB
Anti-JAK1
Rabbit affinity isolated antibody. Application: IB
Anti-JAK 1 [pYpY1022/1023]
Rabbit affinity isolated antibody. Applications: IB, IHC
Anti-JAK2
Rabbit affinity isolated antibody. Application: IB
Anti-JAK 2 [pYpY1007/1008]
Rabbit affinity isolated antibody. Applications: IB, IHC
Anti-JAK3 C-terminal
Rabbit IgG fraction of antiserum. Application: CH
Monoclonal Anti-JAK3
(Clone B32-32) Mouse purified immunoglobulin; Isotype IgG2b.
Application: IP
Anti-PYK2 (CAK-β)
Rabbit affinity isolated antibody. Applications: IB, IP, IHC
Anti-Phospho-Src [pTyr215]
Rabbit affinity isolated antibody. Application: IB
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
37
Protein
Tyrosine Kinases
PROTEIN TYROSINE KINASES
Antibodies to Protein Tyrosine Kinases (continued)
S 1940
Anti-Phospho-Src [pTyr418]
Rabbit affinity isolated antibody. Application: IB
Anti-Phospho-Src [pTyr529]
Rabbit affinity isolated antibody. Application: IB
Anti-Fibroblast Growth Factor
Receptor-1 (FGFR-1)
Rabbit affinity isolated antibody. Applications: IB, IP, IHC
F 0300
Anti-Fibroblast Growth Factor
Receptor-2 Cytoplasmic (FGFR-2)
Rabbit affinity isolated antibody. Applications: IB, IP, IHC
F 6796
Anti-Fibroblast Growth Factor
Receptor-2 Extracellular (FGFR-2)
Rabbit affinity isolated antibody. Applications: IB, IP, IHC
F 0425
Anti-Fibroblast Growth Factor
Receptor-3 Cytoplasmic (FGFR-3)
Rabbit affinity isolated antibody. Applications: IB, IP, IHC
F 3922
Anti-Fibroblast Growth Factor
Receptor-3 Extracellular (FGFR-3)
Rabbit affinity isolated antibody. Applications: IB, IHC
E 2760
Anti-Epidermal Growth Factor
Receptor
(Clone 29.1) Mouse, ascites fluid, isotype IgG1. Application: may be
used to purify EGF receptor for structural and functional studies.
E 3138
Anti-Epidermal Growth Factor
Receptor
(Clone F4) Mouse, ascites fluid; isotype IgG1. Applications: IB, IP, IHC, EL
E 8767
Anti-c-erbB-3
(Clone RTJ1) Mouse, ascites fluid; isotype IgM. Application: IHC
I 6031
Anti-Phospho-IRS-1 [p-Ser616]
Rabbit affinity isolated antibody. Applications: IB, IHC
I 7151
Anti-Insulin-Like Growth Factor-1
Receptor (IGF-1 R)
Goat affinity isolated antibody. Applications: IB, EL
I 6068
Anti-Insulin Receptor, α-subunit
Chicken fractionated antiserum. Application: CH
I 6153
Anti-Insulin Receptor, β-subunit
Rabbit IgG fraction of antiserum. Applications: IB, IP, IC
I 7153
Anti-Insulin Receptor Substrate 1 (IRS-1) Rabbit affinity isolated antibody. Applications: IB, IP
I 7278
Anti-Insulin Receptor Substrate 2 (IRS-2) Rabbit IgG fraction of antiserum. Applications: IB, IP
w
Ne
S 2065
w
Ne
F 5421
w
Ne
w
Ne
38
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
Calcium/CaM
CAMKI
Chin, D., et al., J. Biol. Chem., 272, 31235-31240 (1997).
Jayanthi, L.D., et al., Br. J. Pharmacol., 129, 465-470 (2000).
Matsushita, M. and Nairn, A.C., J. Biol. Chem., 274, 10086-10093 (1999).
Wu, G-Y, et al., Proc. Natl. Acad. Sci. USA., 98, 2808-2813 (2001).
CAMKII
Bennecib, M., et al., FEBS Lett., 490, 15-22 (2001).
Feinmesser, R.L., et al., J. Biol. Chem., 274, 16168-16173 (1999).
Hughes, K., et al., J. Biol. Chem., 276, 36008-36013 (2001).
Liang, F., et al., Exp. Brain Res., 110, 163-174 (1996).
Singla, S.I., et al., J. Biol. Chem., 276, 29353-29360 (2001).
CAMKII γ
Aronowski, J., et al., J. Cereb. Blood Flow Metab., 20, 343-349 (2000).
Bui, J.D., et al., Cell, 100, 457-467 (2000).
Fahrmann, M., et al., Eur. J. Biochem., 266, 1036-1042 (1999).
Kwiatkowski, A.P., et al., Arch. Biochem. Biophys., 378, 377-383 (2000).
Stevens, I., et al., J. Biochem. (Tokyo), 129, 551-560 (2001).
CAMKIV
Ho, N., et al., J. Neurosci., 20, 6459-6472 (2000).
Jang, M.K., et al., J. Biol. Chem., 276, 20005-20010 (2001).
Wu, J.Y., et al., Endocrinology, 141, 4777-4783 (2001).
Wu, Y., et al., Proc. Natl. Acad. Sci. USA, 98, 2877-2881 (2001).
Yu, C.T., et al., J. Immunol., 166, 284-292 (2001).
CAMKK
Anderson, K.A., et al., J. Biol. Chem., 273, 31880-31889 (1998).
Soderling, T.R., Trends Biochem. Sci., 24, 232-236 (1999).
Tokumitsu, H., et al., J. Biol. Chem., 274, 15803-15810 (1999).
MLCK
Katoh, K., Am. J. Physiol. Cell Physiol., 280, 1669-1679 (2001).
Lazar, V. and Garcia, J.G., Genomics, 57, 256-267 (1999).
Pfitzer, G.J., Appl. Physiol., 91, 497-503 (2001).
Cyclins
Garrett, S., et al., Mol. Cell. Biol., 21, 88-99 (2001).
Kaldis, P., et al., J. Biol. Chem., 275, 32578-32584 (2000).
Poon, R.Y.C., et al., J. Biol. Chem., 271, 13283-13291 (1996).
Smits, V.A.J., et al., J. Biol. Chem., 275, 30638-30643 (2000).
PKA/PKG
Hancock, J.T., Cell Signaling, Addison Wesley Longman Harlow, pp. 6166 (1997).
Pedram, A., et al., J. Biol. Chem., 275, 7365-7372 (2000).
PKB/Akt
Brenneisen, P., et al., J. Biol. Chem., 275, 4336-4344 (2000).
Hayashi, M., et al., J. Biol. Chem., 276, 8631-8634 (2001).
Kobayashi, T., et al., Biochem. J., 339, 319-328 (1999).
Kobayashi, T., et al., Biochem. J., 344, 189-197 (1999).
Protein Kinase C
Diaz-Meco, M.T. et al., Mol. Cell. Biol., 21, 1218-1227 (2001).
Flynn, P., et al., J. Biol. Chem., 275, 11064-11070 (2000).
Gao, T., et al., J. Biol. Chem., 276, 19588-19596 (2001).
Gill, P.K., et al., Eur. J. Biochem., 268, 4151-4157 (2001).
Jun, J.Y., et al., Am. J. Physiol. Cell. Physiol., 281, C857-C864 (2001).
Newton, A.C., Curr. Biol., 9, 161-167 (1997).
Parekh, D.B., et al., EMBO J., 19, 496-503 (2000).
Perez, J.L., et al., J. Neurosci., 21, 5417-5428 (2001).
Webb, B.L.J., et al., Br. J. Pharmacol., 130, 1433-1452 (2000).
Yang, Z., et al., Biochem. Biophys. Res. Commun., 286, 372-375 (2001).
MAPK
Chao, T.-H., et al., J. Biol. Chem., 274, 36035-36038 (1999).
Chen, G., et al., J. Biol. Chem., 275, 38973-38980 (2000).
Cheng, M., et al., J. Biol. Chem., 271, 12057-12062 (1996).
Deak, M., et al., EMBO J., 17, 4426-4441 (1998).
Fleming, Y., et al., Biochem. J., 352, 145-154 (2000).
Goedert, M., et al., EMBO J., 16, 3563-3571 (1997).
Hoover, H.C., et al., J. Biol. Chem., 275, 23825-23833 (2001).
Lee, J.K. et al., Brain Res. Mol. Brain Res., 66, 133-140 (1999).
Lim, H.Y., et al., Biochem. Biophys. Res. Commun., 285, 77-83 (2001).
Ludwig, S., et al., Mol. Cell Biol., 16, 6687-6697 (1996).
Mody, N., et al., FEBS Lett., 502, 21-24 (2001).
New, L., et al., EMBO J., 17, 3372-3384 (1998).
Pugazhenthi, S., et al., J. Biol. Chem., 274, 27529-27535 (1999).
Ryder, J.W., et al., J. Biol. Chem., 275, 1457-1462 (2000).
Sun, W., et al., J. Biol. Chem., 276, 5093-5100 (2001).
Sutherland, C.L., et al., J. Immunol., 162, 4720-4730 (1999).
Vacratsis, P.O. and Gallo, K.A., J. Biol Chem., 275, 27893-27900 (2001).
Werz, O., et al., Proc. Natl. Acad. Sci. USA, 97, 5261-5266 (2000).
Zhang, Y., et al., J. Biol. Chem., 276, 14572-14580 (2001).
Tyrosine
References/
Abbreviations
REFERENCES
Bcr/Abl
Gesbert, F., et al., J. Biol. Chem., 275, 39223-39230 (2000).
Heisterkamp, N., et al., Blood, 15, 2226-2232 (2000).
Lim, Y.M., et al., Proc. Natl. Acad. Sci. USA, 97, 12233-12238 (2000).
Wu, Y. et al., Oncogene, 161, 141-146 (1998).
BTK
Kurosaki, T. and Kurosaki, M., J. Biol. Chem., 272, 15595-15598 (1997).
Nore, B.F., et al., Eur. J. Immunol., 30, 145-154 (2000).
Qiu, Y. and Kung, H.J., Oncogene, 20, 5651-5661(2000).
Tomlinson, M.G., et al., Immunology, 2, 2-19 (2001).
Tomlinson, M.G., et al., J. Biol. Chem., 274, 13577-13585 (1999).
CSK
Cloutier, J.-F. and Veillette, A., EMBO J., 15, 4909-4918 (1996).
Sondhi, D. and Cole, P.A., Biochemistry, 38, 11147-11155 (1999).
Superti-Furga, G., et al., EMBO J., 12, 2625-2634 (1993).
Zrihan-Licht, S., et al., J. Biol. Chem., 273, 4065-4072 (1998).
EGFR
Hognason, T., et al., FEBS Lett., 491, 9-15 (2001).
Keating, K.E., et al., Oncogene, 32, 4281-4290 (2001).
Kumagai, T., et al., Proc. Natl. Acad. Sci. USA, 98, 5526-5531 (2001).
Lu, Z., et al., Mol. Cell Biol., 21, 4016-4031 (2001).
Zhu, X.F, et al., Cancer Lett., 169, 27-32 (2001).
EMT (ITK)
Gibson, S., et al., J. Biol. Chem., 271, 7079-7083 (1996).
Libo, Y., et al., J. Biol. Chem., 272, 13033-13039 (1997).
Schaeffer, E.M., et al., Science, 284, 638-641 (1999).
Yang, W-C., et al., J. Biol. Chem., 274, 607-617 (1999).
FAK
Cary, L.A. and Guan, J-L., Frontiers Biosci., 4, D102-D113 (1999).
Nakamura, K., et al., Oncogene, 21, 2626-2635 (2001).
Reiske, H.R., et al., FEBS Lett., 486, 275-280 (2000).
Sonoda, Y., et al., J. Biol. Chem., 275, 16309-16315 (2000).
Yamakita, Y. et al., J. Cell Biol., 144, 315-324 (1999).
FER (TYK3)
Arregui, C., et al., J. Cell Biol., 149, 1263-1274 (2000).
Hao, Q.L., et al., Mol. Cell Biol., 9, 1587-1593 (1989).
Kapus, A., et al., J. Biol. Chem., 275, 32289-32298 (2000).
FES A93
Jianze, Li and Smithgall, T.E., J. Biol. Chem., 273, 13828-13834 (2000).
Rogers, J.A., et al., J. Biol. Chem., 271, 17519-17525 (1996).
Yates, K.E., et al., Oncogene, 10, 1239-1242 (1995).
FGFR
Arbeit, J.M., et al., Oncogene, 13, 1847-1857 (1996).
Dell’Era, P., et al., Mol. Biol. Cell., 10, 23-33 (1999).
Guillonneau, X., et al., J. Biol. Chem., 273, 22367-22373 (1998).
Tannheimer, S.L., et al., Breast Cancer Res., 2, 311-320 (2000).
FYN
Hansen, K., et al., FEBS Lett., 409, 195-200 (1997).
Hunter, A.J., et al., J. Immunol., 159, 4806-4814 (1997).
Marie-Cardine, A., et al., J. Biol. Chem., 272, 16077-16080 (1997).
Salojin, K., et al., J. Exp. Med., 186, 887-897 (1997).
Wong, J., et al., Mol. Cell Biol., 18, 2855-2866 (1998).
Hck
Johnson, T.M., et al., J. Biol. Chem., 275, 33353-33364 (2000).
Ziegler, S.F., et al., Mol. Cell. Biol., 7, 2276-2285 (1987).
JAKs
Carter-Su, C., et al., Proc. Nat. Acad. Sci. USA, 91, 5232-5236 (1994).
Gozalo-Sanmillan, S., et al., J. Immunol., 166, 727-730 (2001).
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
39
References/
Abbreviations
R E F E R E N C E S / A B B R E V I AT I O N S
Murakami, Y., et al., J. Cell Physiol., 175, 220-228 (1995).
Parganas, E., et al., Cell, 93, 385-395 (1998).
Zhang, J-G., et al., Proc. Natl. Acad. Sci. USA, 96, 2071-2076 (1999).
Lck
Denny, M.F., et al., Mol. Cell Biol., 20, 1426-1435 (2000).
Shan, X. and Wange, R.L., J. Biol. Chem., 274, 29323-29330 (1999).
Straus, D.B., et al., J. Biol. Chem., 271, 9976-9981 (1996).
Sundvold, V., et al., J. Immunol., 165, 2927-2931 (2000).
Wong, J., et al., Mol. Cell Biol., 18, 2855-2866 (1998).
LYN
Falati, S., et al., Blood, 94, 1648-1656 (1999).
Quek, L.S., et al., Blood, 96, 4246-4253 (2000).
Sotirellis, N., et al., J. Biol. Chem., 270, 29773-29780 (1995).
Wilson, B.S., et al., J. Cell Biol., 276, 645-658 (2001).
PDGFR
Berrozpe, G, et al., Blood, 94, 2658-2666 (1999).
Bioukar, E.B., et al., J. Biol. Chem., 274, 21457-21463 (1999).
Heldin, C.H., et al., Biochim. Biophys. Acta, 1378, F79-113 (1998).
Klinghoffer, R.A., et al., Mol. Cell, 7, 343-354 ( 2001).
Miyake, S., et al., J. Biol. Chem., 274, 16619-16628 (1999).
Sachiko, M., et al., Proc. Natl. Acad. Sci. USA, 95, 7927-7932 (1998).
Wang J., et al., Mol. Cell. Biol., 19, 6217-6228 (1999).
c-Src
Benard, O., et al., J. Biol. Chem., 276, 4554-4563 (2001).
Blake, R.A., et al., Mol. Cell. Biol., 20, 9018-9027 (2000).
Fincham, V.J., et al., Mol. Cell. Biol., 20, 6518-6536 (2000).
Suzuki-Inoue, K., et al., J. Biol. Chem., 276, 1643-1652 (2001).
SYK
Baba, Y., Proc. Natl. Acad. Sci. USA, 98, 2582-2586 (2000).
Chu, D.H., et al., J. Immunol., 163, 2610-2620 (1999).
Veale, M., et al., J. Biol. Chem., 274, 28427-28435 (1997).
Wong, J., et al., Mol. Cell Biol., 18, 2855-2866 (1998).
Yankee, T.M., et al., J. Immunol., 163, 5827-5835 (1999).
TEC
Schaeffer, E.M., et al., Science, 284, 638-641 (1999).
Sommers, C.L., et al., J. Exp. Med., 190, 1427-1438 (1999).
YES
Chen, R., et al., J. Biol. Chem., 276, 31858-31862 (2001).
Feshchenko, E.A., et al., J. Biol. Chem., 273, 8323-8331 (1998).
Marchetti, D., et al., Oncogene, 25, 3253-3260 (1998).
Tang, H., et al., J. Biol. Chem., 275, 389-396 (2000).
ZAP-70
Brockdorff, J., et al., Eur. J. Immunol., 29, 2539-2550 (1999).
Ottoson, N.C., et al., J. Immunol., 167, 1857-1861 (2001).
van Leeuwen, J.E., et al., Mol. Cell Biol., 19, 6652-6664 (1999).
Wu, J. et al., J. Exp. Med., 185, 1877-1882 (1997).
Williams, B.L., et al., EMBO J., 18, 1832-1844 (1999).
A B B R E V I AT I O N S / D E S C R I P T I O N S
ATF-1, 2: Activating transcription factor 1, 2
BAD:
Bcl2-antagonist of cell death
4E-BP1:
eIF-4E Binding protein-1
CAK/Pyk2: Proline-rich/Ca2+-activated tyrosine kinase
c-Cbl:
Oncogene
CH:
Chemiluminescence
CLIC3:
Chloride intracellular channel 3
CNS:
Central nervous system
CNTF:
Ciliary neurotrophic factor
CREB:
cAMP Responsive element binding protein
Crk:
Adaptor protein
CSBP:
Stress-activated mitogen-activated protein kinase p38
CSK:
COOH-terminal Src kinase
CT-1:
Cardiotropin-1
DAG:
Diacylglycerol
DARPP-32: Dopamine- and cAMP-regulated phosphoprotein, 32 kDa
DB:
Dot blotting
eIF-4E:
Eukaryotic initiation factor 4E
EL:
Elisa
Elk1:
ETS oncogene family member
GP30:
Transmembrane protein
Grb2:
Adaptor protein
Hsp27:
Heat shock protein 27
IB:
Immunoblotting
IC:
Immunocytochemistry
IF:
Immunofluorescence
IHC:
Immunohistochemistry
ILK:
Integrin-linked kinase
ILs:
Interleukins – 2, 3, 4, 6, 9, 11, 13, 15
IP:
Immunoprecipitation
IRS1, 2:
Insulin receptor substrate 1, 2
IS:
Immunostaining
JunD:
Jun oncogene family member
LIF:
Leukemia inhibitory factor
LSP-1:
5-LO lymphocyte specific protein
MAP2:
Microtubule associated protein 2
MARCKS: Myristoylated alanine-rich C kinase substrate
MDR:
Multi-drug resistance
MEF2C:
Myocyte enhancer factor 2C
Mxi2:
Splice variant of p38
40
Myc:
MyH:
MyoD:
NCK:
NFkB:
NF-H:
NF-M:
NRG2,3:
NTAK:
OSM:
PI3K:
PICK1:
PKD:
PKK:
PKN:
PLA2:
PLCγ:
PS:
P-TEFB:
RACK:
Rb:
RGS3, 4:
RIA:
RNAPII:
RP:
SH2/SH3:
Shc:
Shp2:
SRF:
STATs:
STK1:
TAK1:
Tao1, 2:
τ:
τ PKII:
TCR:
TGFα:
TK:
ZIP/p62:
Oncogene
Myosin, heavy
Myogenic differentiation antigen
Adaptor protein
Nuclear factor k B
Neurofilament H
Neurofilament M
Neuregulin 2, 3
Neural- and thymus-derived activator for ErbB kinases
Oncostatin M
Phosphoinositide 3-OH kinase
Protein interacting with protein kinase C 1
Protein kinase D
Protein kinase C-associated kinase
Protein kinase N
Phospholipase A2
Phospholipase C γ
Phosphoserine
Positive transcription elongation factor b
Receptors for activated C kinase
Retinoblastoma
Regulators of G protein signaling 3, 4
Radioimmunoassay
RNA polymerase II
Arginine-proline region
Src homology domains
Adaptor protein
Protein tyrosine phosphatase 2C
Serum response factor
Signal transducers and activators of transcription
Serine/threonine protein kinase
TGF-β-activated kinase
Thousand and one amino acid kinase
tau
tau protein kinase II
T-cell antigen receptor
Transforming growth factor alpha
Tyrosine kinase
Binding protein for PKC
Order: 1.800.325.3010 • Technical Service: 1.800.325.5832 • sigma-aldrich.com/cellsignaling
SIGMA-ALDRICH SALES OFFICES
Argentina
SIGMA-ALDRICH DE
ARGENTINA, S.A.
Tel: (54-11) 4807-0321
Fax: (54-11) 4807-0346
Australia
SIGMA-ALDRICH PTY., LIMITED
Free Tel: 1 800 800 097
Free Fax: 1 800 800 096
Tel: 61-2 8853 5555
Fax: 61-2 8853-5500
Austria
SIGMA-ALDRICH Handels GmbH
Tel: 01-605 81 10
Fax: 01-605 81 20
Belgium
SIGMA-ALDRICH N.V./S.A.
Free Tel: 0800-147.47
Free Fax: 0800-147.45
Tel: 03 899.13.01
Fax: 03 899.13.11
Germany
SIGMA-ALDRICH Chemie GmbH
Free Tel: 0800-51 55 000
Free Fax: 0800-64 90 000
Telefon: +49(0)89-65 13-0
Telefax: +49(0)89-65 13-11 69
Greece
SIGMA-ALDRICH (o.m.) Ltd.
Tel: 30-1-9948010
Fax: 30-1-9943831
Hungary
SIGMA-ALDRICH Kft
Tel Ingyenes: 06-80-355-355
Fax Ingyenes: 06-80-344-344
India
SIGMA-ALDRICH CORP.
Bangalore Location:
Tel: (080) 852 4222
Fax: (080) 852 4214
New Delhi Location
Tel: (011) 689 9826
Tel: (011) 689 7830
Fax: (011) 689 9827
Brazil
SIGMA-ALDRICH Química
Brasil Ltda.
Tel: (011) 231 1866
Fax: (011) 257 9079
Canada
SIGMA-ALDRICH CANADA LTD.
Free Tel: 800-565-1400
Free Fax: 800-265-3858
Tel: 905-829-9500
Fax: 905-829-9292
Ireland
SIGMA-ALDRICH IRELAND LTD.
Free Tel: 1-800-200-888
Free Fax: 1-800-600-222
Tel: 01-404-1900
Fax: 01-404-1910
Israel
SIGMA-ALDRICH ISRAEL LTD.
Free Tel: 1-800-70-2222
Tel: (08) 9484-222
Fax: (08) 9484-200
Czech Republic
SIGMA-ALDRICH s.r.o.
Tel: 02/21761310
Fax: 02/21763300
Denmark
SIGMA-ALDRICH DENMARK A/S
Tel: 43 56 59 10
Fax: 43 56 59 05
Finland
SIGMA-ALDRICH Finland/YA-Kemia
OY
Tel: (09) 350-9250
Fax: (09) 350-92555
France
SIGMA-ALDRICH Chimie S.à.r.l.
Tél Numéro Vert: 0800 21 14 08
Fax Numéro Vert: 0800 03 10 52
Italy
SIGMA-ALDRICH S.r.l.
Numero Verde: 800-827018
Telefono: 02-33417310
Fax: 02-38010737
Japan
SIGMA-ALDRICH JAPAN K.K.
Tokyo Tel: (03) 5821 3594
Tokyo Fax: (03) 5821 3590
Korea
SIGMA-ALDRICH KOREA
Toll Free Tel: 080-023-7111
Toll Free Fax: 080-023-8111
Tel: 02-783-5211
Fax: 02-783-5011
Malaysia
SIGMA-ALDRICH (M) Sdn. Bhd
Free Tel: 60 3 782 4181
Fax: 60 3 782 4067
Mexico
SIGMA-ALDRICH Química
S.A. de C.V.
Free Tel: 01-800-007-5300
Fax: 01-800-712-9920
The Netherlands
SIGMA-ALDRICH Chemie BV
Tel Gratis: 0800-0229088
Fax Gratis: 0800-0229089
Tel: 078-6205411
Fax: 078-6205421
New Zealand
SIGMA-ALDRICH PTY., LIMITED
Free Tel: 0800 936 666
Free Fax: 0800 937 777
Norway
SIGMA-ALDRICH NORWAY AS
Tel: 23176060
Fax: 23176050
Singapore
SIGMA-ALDRICH PTE., LTD.
Tel: (65) 271 1089
Fax: (65) 271 1571
South Africa
SIGMA-ALDRICH S.A. (Pty.) Ltd.
Tel: 0800-110075
Fax: 0800-110079
Spain
SIGMA-ALDRICH QUÍMICA S.A.
Free Tel: 900 101376
Free Fax: 900 102028
Tel: 91-6619977
Fax: 91-6619642
Sweden
SIGMA-ALDRICH SWEDEN AB
Tel: 020-35 05 10
Fax: 020-35 05 22
Outside Sweden Tel: 46-8-742 4200
Outside Sweden Fax: 46-8-742 4243
Switzerland
SIGMA-ALDRICH Chemie
Swiss Free Call: 0800 80 00 80
Tel: 081-755 27 21
Fax: 081-755 28 40
United Kingdom
SIGMA-ALDRICH COMPANY LTD.
Free Tel: 0800 717181
Free Fax: 0800 378785
Tel: 01202 733114
Fax: 01202 715460
Poland
SIGMA-ALDRICH Sp. z o.o
Tel: 061 823 24 81
Fax: 061 823 27 81
United States
SIGMA-ALDRICH
Toll-free Tel.: 800-325-3010
Call Collect: 314-771-5750
Toll-Free Fax: 800-325-5052
Fax: 314-771-5757
Portugal
SIGMA-ALDRICH QuÍmica, S.A.
Free Tel: 0800 20 21 80
Free Fax: 0800 20 21 78
Russia
SIGMA-ALDRICH RUSSIA
TechCare Systems, Inc.
(TechMedBioChem)
Tel: (095) 975-3321
Fax: (095) 975-4792
Internet:
sigma-aldrich.com
Quantity Pricing/
Standing Orders:
800-521-8956 or
314-771-5765 Ext. 3702
Development/
Manufacturing Inquiries:
800-336-9719 • 314-534-4900
sigma-aldrich.com/cellsignaling
®
®
P.O. Box 14508
St. Louis, MO 63178 USA
Return Service Requested
!
ailable
v
A
w
No
88
o. S 81
N
.
d
o
Pr
sigma-aldrich.com/cellsignaling
Technical Service 1-800-325-5832
Development/Manufacturing Inquiries
Sigma-Aldrich Fine Chemicals 1-800-336-9719
Order/Customer Service
1-800-325-3010
Fax 1-800-325-5052
World Headquarters • 3050 Spruce St., St. Louis, MO 63103 • (314) 771-5750 • sigma-aldrich.com
The
SIGMA-ALDRICH
Family
EJP
0101
500174-853
Biochemicals and
Reagents for Life
Science Research
Organics and
Inorganics for
Chemical Synthesis
Specialty Chemicals
and Analytical
Reagents for Research
Laboratory Chemicals
and Reagents for
Research and Analysis
Chromatography
Products for Analysis
and Purification
©2001 Sigma-Aldrich Co. Printed in USA Sigma brand products are sold through Sigma-Aldrich, Inc.
Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their
particular use. Additional terms and conditions may apply. Please see reverse side of the invoice or packing slip. SIGMA and - are registered trademarks of Sigma-Aldrich Biotechnology LP. Riedel-de
Haën®: trademark under license from Riedel-de Haën GmbH.