Chapter 2
advertisement

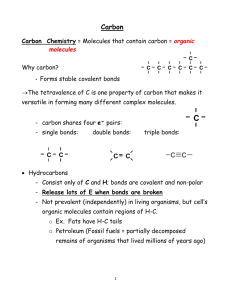
The Chemical Basis of Life All organisms are made up of water, inorganic ions, small molecules (77% by weight) and macromolecules (23% by weight in E.coli). white=hydrogen red=oxygen gray=carbon yellow=phosphorus blue=nitrogen green=sulfur Chemical Bonds Atoms that make up a molecule are joined together by Covalent Bonds, where electron pairs are shared between atoms. Atoms are most stable with a full outer electron shell The number of bonds formed is determined by the number of electrons needed to fill outer shell Bond formation is accompanied by energy release Breaking these covalent bonds requires energy Examples: C-C, C-H or C-O covalent bonds require large amounts of energy to break, so these bonds are stable under most conditions Fig. 2-1, Lodish et al., Mol. Cell Biol., 4th edition. WH Freeman and Co., and Sumanas, Inc., 2000 Fig 2.1 Arrangement of electrons in common atoms found in biological molecules Atoms can be joined by single bonds, or by bonds in which 2 pairs of electrons are shared - double bond (O2); if 3 pairs are shared triple bond (N2) The type of bond determines the shape of the molecular - atoms joined by a single bond can rotate relative to one another; double & triple bonds cannot . The double bond between the 2 carbon atoms in Ethylene make the molecule rigid. Fig. 2-3. Lodish et al., Mol. Cell Biol., 4th edition. WH Freeman and Co., and Sumanas, Inc., 2000 . Polar and Non-polar molecules In H2O (water), the - O-H bonds are polarized (one atom [O] is partially negative; the other [H] is partially positive); water is a polar molecule – and has an asymmetric charge distribution. The water molecule has 2 polar –O-H bonds and therefore has a dipole moment [a + charge separated from an equal – charge]. This means that the area around the oxygen atom has a mostly negative change and the areas around the hydrogen atoms have a mostly positive charge. Biologically important polar molecules have one or more electronegative atoms - usually O, N, S and/or P). Because O is more electronegative than H, shared electrons spend more time near the O atom than the H atom. Molecules without electronegative atoms & polar bonds (ex: C & H) are nonpolar. These molecules are relatively inert. Many biological molecules that we will study (proteins, phospholipids, others) have both polar & nonpolar regions. This influences what kind of bonds they can participate in. δ, represents a partial charge Fig. 2-5. Lodish et al., Mol. Cell Biol., 4th edition. WH Freeman and Co., and Sumanas, Inc., 2000 1 Noncovalent Bonds Noncovalent bonds are very important in interactions between molecules or different parts of a large biological molecule. Fig 2.7, Hydrogen bond formation between neighboring water molecules They are weaker than covalent bonds (require less energy to break or make). Individual noncovalent bonds have low energies (~1 5 kcal/mole) and are easily broken & reformed. They depend on attractive forces between positively & negatively charged regions within same molecule or on two molecules that are VERY close close to each other. When many of them act together (in DNA, protein, etc.), attractive forces add up & provide structure with stability. (Cooperativity or “strength in numbers”) Because they are weaker they are transient (temporary or short lived) which explains why noncovalent bonds mediate the dynamic interactions among molecules within the cell. Noncovalent Bonds Understanding noncovalent bonds and how and when they work will help us to understand how one molecule interacts with other molecules inside cells. For molecules to affect each other, they must be close enough to form noncovalent bonds. There are 4 kinds of noncovalent bonds or interactions. 1. Ionic bonds (also called salt bridges) result from transfer of electrons from 1 atom to another, leading to atoms with positive & negative charges that attract each other. These weak bonds can hold molecules together (ex: protein to DNA). Also important between oppositely charged groups of a single large molecule, such as inside protein centers where water is not present. 3. Hydrophobic (water-fearing) interactions - not true bonds since not usually thought of as attraction ,rather than a chemical bond between hydrophobic molecules Molecules with nonpolar covalent bonds lack charged regions that can interact with water molecules & so are insoluble in water Hydrophobic molecules form into aggregates minimizing exposure to water or other polar surroundings (ex: fat on chicken soup). These interactions are important inside proteins and inside membranes. They occur between hydrophobic side (R) groups of amino acids in the protein interior away from H2O. They are also important inside membranes where they occur among the nonpolar lipid “tails” of phospholipid molecules. 2. Hydrogen (H) bonds - hydrophilic (water-loving); enhance solubility of molecules in water, & interactions with water. When H is bonded to an electronegative atom (ex: O or N), the shared electron pair goes toward electronegative atom, so H is partially positive. H can be shared between two electronegative atoms. H bonds occur between most polar molecules. H bonds are important in determining structure & properties of water, also between polar groups & large biological molecules (like DNA) Strong collectively, but weak individually (2 - 5 kcal/mole in aqueous solutions) Fig. 2.3 Noncovalent ionic bonds between protein and DNA, noncovalent H bonds between the 2 strands of the DNA double helix. 2 4. van der Waals interactions (forces) - hydrophobic groups can form weak bonds with each other based on electrostatic interactions. More than 1 kind of noncovalent bond usually occurs between 2 molecules When 2 molecules are very very close together, if there is a temporary shift in charge, then transient charge separations (dipoles) result. This can be enough to attract 2 molecules to each other. These interactions are VERY weak (0.1 - 0.3 kcal/mole) & very sensitive to distance; molecules must be close together & have complementary shapes that will permit the 2 molecules to closely approach each other. These interactions are important biologically, for example in interactions between antibodies and viral antigens , and between enzymes and substrates. Acids, Bases and Buffers Acid - a molecule able to release (or donate) a hydrogen ion. Whenever a hydrogen atom loses an electron, a proton dissociates & is released. A proton can combine with other molecules to form H3O+, H2O, etc. Base - any molecule capable of accepting a hydrogen ion (proton). Amphoteric molecule - a molecule that can serve as both an acid & a base (usually has both a positive & negative charge); water and amino acids are examples. pH (measure of H+ concentration) = - log10 [H+] In pure water, [H+] = [OH-] = ~10-7 M, or pH=7 Biological processes are very sensitive to pH changes since pH affects the ionic state of biological molecules. Drawing of theoretical interactions between 2 proteins: 2 ionic bonds, 1 H bond, 1 large combination of hydrophobic and van der Waals interactions. This is the situation for most molecular interactions and provides binding specificity. Fig. 2-17. Lodish et al., Mol. Cell Biol., 4th edition. WH Freeman and Co., and Sumanas, Inc., 2000 Under physiological conditions, amino acid side (R) groups are charged (ex: -COOH becomes -COO- and -NH2 becomes -NH3+). Even small pH changes can disrupt shape & activity of an entire protein, messing up biological reactions Buffers minimize pH fluctuations. They bind or release H+ & OH- ions depending on conditions, thus protecting organisms & their cells. Example: pH of fluid within the cell is regulated by phosphate buffer system (H2PO4- & HPO4-2) Excess H+ ions bind HPO4-2; excess OH- ions neutralized by protons derived from H2PO4 Intracellular fluid stays at pH 7.4 Biological molecules: importance of the carbon atom. Fig 2.9 Functional Groups – atoms that behave as a group and help give organic molecules their properties. Examples: Hydroxyl group - —OH; aquires charge —OMethyl group - —CH3 Carboxyl group - —COOH; acquires charge —COOSulfhydryl group - —SH; react to form disulfide bonds in polypeptides Carbon atoms can form covalent bonds with as many as 4 other carbon atoms, or can form double bonds. Steroids like cholesterol include rings. The -OH group provides a small hydrophilic end. In membranes the larger, hydrocarbon portion of cholesterol increases membrane fluidity. Amino group - —NH2; acquires charge —NH3+ Phosphate group - —H2PO3; acquires charge —PO3-2 3 Four families of biological molecules and macromolecules A. Amino acids and proteins B. Carbohydrates (CH2O)n Energy reserves C. Lipids – fats, fatty acids, steroids, phospholipids D. Nucleotides and nucleic acids Fig. 2.11 Fig 2.10, Monomers and Polymers of Macromolecules Fig. 2.17, Three polysaccharides with identical sugar monomers but different shapes and properties. Glucose subunits in 3 different types of linkages. Fig. 2.12, The Structures of Sugars. Sugar (monomers) can be joined together by covalent glycosidic bonds to form disaccharides (ex: sucrose) or short chains called oligosaccharides. Polysaccharides are polymers of sugar unit monomers joined by glycosidic bonds. Fig. 2.19, Fats and fatty acids. a-triglyceride, b-stearic acid, c-tristeareate [another triglyceride or neutral fat], dlinseed oil containing 2 different unsaturated fatty acids due to C=C bonds (yellow kinks). Glycogen is branched Starch is helical Cellulose is linear bundles Fig 2.22, Phosphatidylcholine, a phospholipid. It is amphipathic. Nonpolar, dissolve in organic solvents like chloroform but don’t dissolve in water. Fatty acids have a hydrophilic end (ex: glycerol) and a hydrophobic end (HC chains) and so are termed amphipathic. 4 Proteins: General Information Proteins: Building blocks (monomers) amino acids Composed of H, C, O, N & usually S or P; very large macromolecules; polymers of amino acids (the monomers, linked together by peptide bonds) • Traits & functions - more varied role than other molecules in organisms (enzymes, structural or both); carry out almost all cell activities; typical cell has ~10,000 • Catalyze and accelerate rate of metabolic reactions Cytoskeletal elements serve as structural cables, provide mechanical support in & out of cells Hormones, growth factors, gene activators - regulators Membrane receptors & transporters - determine what cell reacts to, what can leave, enter cell Contractile elements - biological movements Antibodies and toxins, Form blood clot • Transport substances from one part of body to another . Composed of H, C, O, N & usually S or P; very large macromolecules; polymers of amino acids (the monomers, linked together by peptide bonds) Proteins have more varied roles than other molecules in organisms. They carry out almost all cell activities; typical cell has ~10,000 • Catalyze and accelerate rate of metabolic reactions • Cytoskeletal elements serve as structural cables, provide mechanical support in & out of cells • Hormones, growth factors, gene activators – regulators • Membrane receptors & transporters - determine what cell reacts to, what can leave, enter cell • Contractile elements - biological movements • Antibodies and toxins, Form blood clot • Transport substances from one part of body to another . Amino acids: 20 unique amino acids, differ in their R groups All 20 have the same general structure Generalized Amino Acid R H O N H C H C Generalized Amino Acid Physiological pH H R O H OH + N C H H R = Radical or Side Group C O Copyright, ©, 2002, John Wiley & Sons, Inc., Karp/CELL & MOLECULAR BIOLOGY 3E Fig ure 2.24 Fig. 2.24 Formation of a peptide bond. Proteins are always synthesized in the amino-to-carboxyl direction. Fig. 2.26 Amino acid structures – 4 types, get their properties from their side R groups: Polar charged Polar uncharged Nonpolar Other Character of amino R groups is very important to protein structure & function; R groups determine what kind of noncovalent bonds can form 2 ways that protein structures are shown. The protein is Ras, its substrate shown in blue, is guanosine diphosphate. On the right is the water-accessible surface: + charges are blue and – charge are red. Charges are not evenly distributed, and the surface is very bumpy. Fig. 3-5. Lodish et al., Mol. Cell Biol., 4th edition. WH Freeman and Co., and Sumanas, Inc., 2000 5 Other amino acids: in addition to the 20 essential amino acids, other amino acids are sometimes found in polypeptides (ex: hydroxyproline, hydroxylysine). From alterations of R groups of the 20 amino acids after their incorporation into polypeptide. [post-translational modification] The changes greatly change properties & function of a protein, increasing or decreasing its solubility or modifying its interaction with other molecules. Why? Conjugated proteins - involve another type of molecule attached covalently or noncovalently to the protein Nucleoproteins - protein + nucleic acids Lipoproteins - protein + lipids Glycoproteins - protein + carbohydrate Various low-molecular-weight materials, like metals & metalcontaining groups, are often attached Protein structure The sequence of amino acids in the protein (polypeptide) determines its structure and all its properties. The amino acid sequence in referred to as the primary structure of the protein. The genome (DNA) contains instructions for the amino acid sequences. Sickle cell anemia – one amino acid change in Hb: valine [nonpolar] instead of glutamic acid [charged, polar] Secondary structure refers to the conformation or shape of parts of the polypeptide chain. These conformations are present in most proteins. The alpha (α) helix and beta (β)-pleated sheet are secondary structures that are stabilized by H bonds between amino acids of the same polypeptide chain. Alpha helices give strength and rigidity to that part of the protein – wool has many alpha helical regions. Beta-pleated sheets give flexibilty and resistance to tensile (pulling) forces. Silk has mostly beta-pleated sheets as its secondary structure. Parallel ÎÎÎÎ ÎÎÎÎ Antiparallel ÎÎÎÎ ÍÍÍÍ Copyright, ©, 2002, John Wiley & Sons, Inc., Karp/CELL & MOLECULAR BIOLOGY 3E Fig ure 2.30 Copyright, ©, 2002, John Wiley & Sons, Inc., Fig. 2.30 Alpha helix. R groups are on the outside of the helix. Some α helices are amphipathic [have both hydrophilic and hydrophobic regions]. Karp/CELL & MOLECULAR BIOLOGY 3E Fig ure 2.31 Fig. 2.31 Beta-pleated sheet. Sheets in the same polypeptide can run either parallel or antiparallel. R groups extend up and down, out of the sheet. Tertiary structure – conformation of the entire protein. Its 3D shape, stabilized by noncovalent bonds. Most intracellular proteins are globular while extracellular and structural proteins are more fibrous. Copyright, ©, 2002, John Wiley & Sons, Inc., Karp/CELL & MOLECULAR BIOLOGY 3E Figure 2.32 Fig. 2.32. Ribbon model of ribonuclease. Arrows indicate N-terminal to C-terminal direction. Green, loops and turns. Blue, disulfide bonds. Loops and turns of the polypeptide chain connect the alpha helices and beta sheet regions of the protein. Copyright, ©, 2002, John Wiley & Sons, Inc., Karp/CELL & MOLECULAR BIOLOGY 3E Fig ure 2.35 Fig. 3.35, Noncovalent bonds maintain protein conformation. 6 In green, an immunoglobulin domain in Neu Protein domains and motifs Domains - proteins are often composed of 2 or more distinct modules or structural units (domains) that fold independently of one another; often these represent parts of the protein that have separate functions In pink, a membranespanning domain Tissue plasminogen activator DOMAINS Schematic diagrams of some proteins, showing their modular or domain nature. The epidermal growth factor domain, EGF in orange Fig. 3-10. Lodish et al., Mol. Cell Biol., 4 edition. WH Freeman and Co., and Sumanas, Inc., 2000 th Motifs are smaller regions of proteins, commonly occurring substructures that are combinations of α helices and β strands Proteins like these with more than 1 domain may have arisen during evolution by fusion of genes coding for different ancestral proteins; each domain once was separate molecule Domain mix-match creates proteins with unique combinations of activities MOTIFS Proteins are dynamic structures and small changes occur within proteins. Internal movements can be studied with NMR, and we see constant thermal motion in molecules. Examples: random, small fluctuations in bond arrangement shifts in H bonds waving of external side chains (R groups) aromatic ring rotation about single bonds (in Phe, Tyr) Figure 2.37 Figure 2.38 The coiled coil [left] motif in myosin. 2 α helices wrapped around each other. The β barrel motif [right] of triose phosphate isomerase, with β strands of the barrel viewed from the side Quaternary Structure & Multiprotein Complexes Quaternary (4°) structure is the linking of polypeptide chains to form multisubunit functional protein via intermolecular R group interactions. Requires more than 1 polypeptide chain. Multiprotein complexes – made up of different proteins, each with a specific function, that become physically associated to form a much larger complex. • Efficient – since proteins are physically associated, product of one enzyme can be passed directly to next enzyme in sequence; prevents dilution in cell's aqueous medium • Some associations are stable; some not • Stabilized by noncovalent bonds A hemoglobin molecule, composed of 2 α-globin chains and 2 βglobin chains joined by non-covalent bands. Each globin can bind 1 O2 molecule. Binding of O2 to 1 polypeptide causes a change in conformation in the other globins that makes them bind O2 more tightly. Each polypeptide binds heme [in red], an iron-containing complex that actually binds the O2. Figure 2.40b Often interactions between proteins in the complex are regulated by changes like phosphate addition to key amino acids. Example: E. coli pyruvate dehydrogenase - 60 polypeptide chains constituting 3 different enzymes; a stable multiprotein complex whose enzyme activities catalyze reaction series connecting 2 metabolic pathways: glycolysis & TCA cycle 7 Figure 2.45 Denaturation and refolding of ribonuclease. Top left, native enzyme containing disulfide bonds (red=S), and many noncovalent bonds not shown. Refolding occurs after the denaturing agents are removed. Figure 2.43 Protein folding – one possible series of steps 1. generation of much of 2° structure (α-helix & β-pleated sheet) 2. soluble protein folding then driven by hydrophobic bonds; nonpolar groups forced into central core 3. Polypeptide is collapsed into compact state (molten globule) that resembles native protein, but molten globules still lack many R group bonds that maintain final 3° structure 4. noncovalent bonds between R groups (tighter packing) & covalent disufide bonds = native state Molecular chaperones Proteins that help other proteins fold properly into their final 3D conformation. Not specific for a single protein. Chaperones can sense misfolded proteins. They appear to work by binding exposed hydrophobic patches on surfaces of partially folded intermediates, preventing them from reaggregating into incorrect structures. They provide an environment that allows folding & provide no information essential for the process, so proteins still selfassemble. Nucleic Acids – the third class of biopolymers Primarily involved in storage & transmission of genetic information May also be structural (rRNA) or catalytic (ribozymes) Nucleotides are their subunits, phosphodiester bonds join nucleotides together, in a 5’-to-3’ orientation Experimental Pathways, Figure 4 Illustration of proposed steps in molecular chaperones GroEL-GroES-assisted folding of a polypeptide. DNA / RNA Differences: 1 5' CH 2 5' CH 2 OH O 1' 3' O OH 4' H H OH H H 2' OH H Deoxyribose OH 4' 1' H H 3' H H 2' OH OH Ribose In DNA the sugar is deoxyribose, in RNA the sugar is ribose All nucleotides have a common structure Fig. 4-1. Lodish et al., Mol. Cell Biol., 4th edition. WH Freeman and Co., and Sumanas, Inc., 2000 DNA / RNA Differences: 2 DNA is usually double-stranded; RNA is almost always singlestranded. 8 Figure 2.48 DNA / RNA Differences: 3 The nitrogen-containing bases in DNA are A, T, G, C; in RNA they are A, U, G, C. No U in DNA, no T in RNA. Purines have 2 rings. Adenine (A) Guanine (G) Pyrimidines have 1 ring. Thymine (T) Uracil (U) Cytosine (C) In double-stranded DNA, a T is always paired with an A, and a C is always paired with a G. Fig. 2.49, Nitrogenous bases in nucleic acids Double-stranded DNA forms a right-handed double helix, due to the geometry of the sugar-phosphate backbone of DNA. The helix has a major groove and a minor groove. Fig. 4-5. Lodish et al., Mol. Cell Biol., 4th edition. WH Freeman and Co., and Sumanas, Inc., 2000 A single strand of DNA containing 3 bases: cytosine (C), adenine (A) , and guanine (G). Fig. 4-3. Lodish et al., Mol. Cell Biol., and Co., and Sumanas, Inc., 2000 4th edition. WH Freeman Each nucleic acid molecule has a 5’-PO4 end and a 3’-OH end. Synthesis is from 5’-to-3’ Sometimes when proteins noncovalently bind to DNA, it causes the DNA to bend. Here, when the TATA box-binding protein binds to DNA it affects the winding and direction of the double helix. Fig. 4-7. Lodish et al., Mol. Cell Biol., 4 edition. WH Freeman and Co., and Sumanas, Inc., 2000 th Denaturation and renaturation of double-stranded DNA molecules (Breaking and reforming noncovalent hydrogen bonds) Fig. 4-8. Lodish et al., Mol. Cell Biol., 4th edition. WH Freeman and Co., and Sumanas, Inc., 2000 RNA can assume complex shapes. A ribosomal RNA from a bacterial small ribosomal subunit. This is a single molecule Figure 2.50a with lots of H bonds (yellow regions). 9