CHE313: Experiment #7: Nucleophilic Substitution Reactions: SN1
advertisement

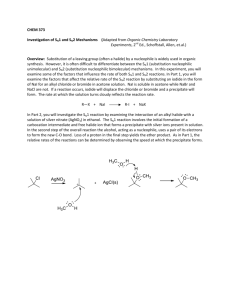
CHE313: Experiment #7: Nucleophilic Substitution Reactions: SN1 vs SN2 Group 1 Br Br Br Br 1-Bromobutane 2-Bromobutane 2-Bromo-2-methyl Propane 1-Bromobenzene Group 2 Cl Cl 1-Chlorobutane Cl Cl 3-Chloro-1-Propene 2-Chloro-2-methyl Propane 1-Chloro-2-methylpropane Group 3 Br Cl Br 1-Bromobutane 2-Bromobutane Cl 1-Chlorobutane 2-Chlorobutane Procedure for SN2 Reactions : 1. 2. 3. 4. Place 100 mg or 100 µL of each compound in 12-separate tubes. Add 1.0 mL of 18% NaI in Acetone to each tube and flick the tubes to mix thoroughly. Record the time it takes for appearance of a precipitate. If no precipitate forms within 5 minutes, place the tube in your sand bath on a setting of 0.5 to 0.75 for 5 minutes and Record the time it takes for the appearance of a precipitate. 5. Rinse the tubes with ethanol and place in the Halogenated Organic Waste. Procedure for SN1 Reactions: 1. Place 100 mg or 100 µL of each compound in 12 separate tubes (that were just rinsed with ethanol). 2. Add 1.0 mL of 1% Ethanolic AgNO3 to each tube and flick the tubes to mix thoroughly. 3. Record the time it takes for appearance of a precipitate. 4. If not precipitate forms within 5 minutes, place the tube in your sand bath on a setting of 0.5 to 0.75 for 5 minutes and Record the time it takes for the appearance of a precipitate. 5. Place the solutions in the Halogenated Organic Waste and clean the tubes. Points to be Addresses in Your Conclusions: Compare the nature of the leaving groups (Cl vs Br) for the tested reactions. Compare the structural effects of the tested compounds (i.e. Primary vs. Secondary vs. Tertiary vs. Allylic). Compare the effect of solvent polarity on the SN1 reaction (Ethanol vs. Acetone). The Effect of Temperature on the Reactions.