Week 3 Nucleophillic Substitutions
advertisement

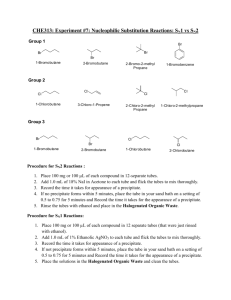
Chemistry 242 Experiment #2 Nucleophillic Substitution Reactions Prior Reading: Bruice, chapter 8 http://www.chemguide.co.uk/mechanisms/nucsubmenu.html http://www.chem.ucalgary.ca/courses/351/Carey/Ch08/ch8-0.html 1 The goal of this experiment is to determine how the structure and nature of the electrophile affects the rate in each of these mechanisms. For example, the rate of an SN1 reaction depends on the carbocation stability and you know that the trend of stability is tertiary > secondary > primary > methyl. You also know that the rate of the SN2 reaction is sensitive to steric hindrance. Do these trends show up in your data? What else does your data show? The alkyl halides to be tested are: 2-bromopropane 1-chlorobutane 2-bromobutane 2-chlorobutane 2-chloro-2-methylpropane iodoethane For the PreLab, you only have to do 2 balanced eqns, one for the SN1 rxn and one for SN2 rxn (any reactant). Procedure Work in pairs, in the hood. One person will mix the reactants and the partner will record data and observations. Before beginning you should give some thought to organizing a table for your work. Draw the molecular structure for each alkyl halide. All test tubes should be clean and dry. If you have to wash them they should be rinsed with a small amount of the reaction solvent. A designated waste container will be provided for the reaction solutions and rinses. Set up a warm water bath (~50 degrees) in the hood using a 100 mL beaker on a hotplate set to low. To follow the rates, it may be easier to react only four test tubes at a time. SN2 reactions using NaI/acetone: - This reagent provides an excellent nucleophile, I , and a nonpolar solvent, which favors a bimolecular (SN2) mechanism. If a reaction occurs, the iodide substitutes for the chloride or bromide producing acetoneinsoluble NaCl or NaBr. Hence the appearance of a white precipitate indicates a positive reaction. Iodoethane should not give a positive reaction here (why not?). 2 Assemble and label six clean, dry test tubes. Add to each the different alkyl halides (0.1 mL). To each test tube add 1 mL of a 1 M solution of NaI in acetone and mix thoroughly. For each tube, note how long after the addition of the NaI solution the first trace of precipitate appears. Watch the tubes to a maximum time of 10 minutes after the addition. If a precipitate does not appear, heat the tube in a warm water bath for an additional 10 minutes and watch for any change. Cover the tubes with aluminum foil. Be careful not to allow the acetone to evaporate. SN1 reactions using AgNO3/ethanol: This reagent contains a polar solvent and a coordinating silver cation, which supports ionization of the halide from the alkyl halide, facilitating a unimolecular (SN1) pathway. The formation of a precipitate indicates a positive reaction between the alkyl halide and ethanolic AgNO 3 due to the formation of ethanolinsoluble AgCl or AgBr. In other words, the leaving group leaves, and is immediately precipitated out with Ag ion. Assemble and label six clean, dry test tubes. To each add a different alkyl halide (0.1 mL). To each test tube add 1 mL of a 0.1 M solution of AgNO3 in ethanol and mix thoroughly. (Avoid skin contact! Silver salts produce long-lived brown stain on your skin). For each tube, note how long after the addition of the AgNO3 solution the first trace of precipitate appears. Watch the tubes to a maximum time of 10 minutes after the addition. If no change occurs after that time, place the tube in the warm water bath and watch for any precipitate formation over the next 10 minutes. Cover the tubes with aluminum foil. Be careful not to allow the ethanol to evaporate. When you are finished, put the empty test tubes in the hood. Be careful to avoid contact and minimize exposure to the alkyl halides. Also avoid getting the silver nitrate solution on your skin. All used solutions to the Halogenated Waste Jar. Post Lab Questions 1. Based on your results, predict how the reaction rates of the following compounds will compare in an SN1 rxn to those that were tested. The best way to answer this is to rank all thirteen molecules from most reactive (#1) to least reactive (#13). There can be ties. For instance, if 2 or more don’t react in SN1, they would be tied for last. 1-bromobutane 1-chloro-2-methylpropane 2-bromo-2-methylpropane 1-chloro-2-methyl-1-propene 2-iodo-2-methylpropane benzylbromide (C7H7Br) bromobenzene (C6H5Br) 2. From the question one list, predict the fastest SN1 reactant and the fastest SN2 reactant. Then sketch the NMR of the two products. If you predict that the same product will be generated for both the fastest SN2 and SN1, sketch the NMR of the second fastest SN2. In other words, no matter what your prediction, you have two NMR sketches. Clearly indicate approximate chemical shift, integration, splitting pattern, and which H’s on the molecule correspond with which peaks on the NMR. Good experimental procedures can be found at http://www.bradley.edu/las/chm/Course/250/Substitution.pdf 3