CHEM 373 Investigation of SN1 and SN2 Mechanisms (Adapted
advertisement
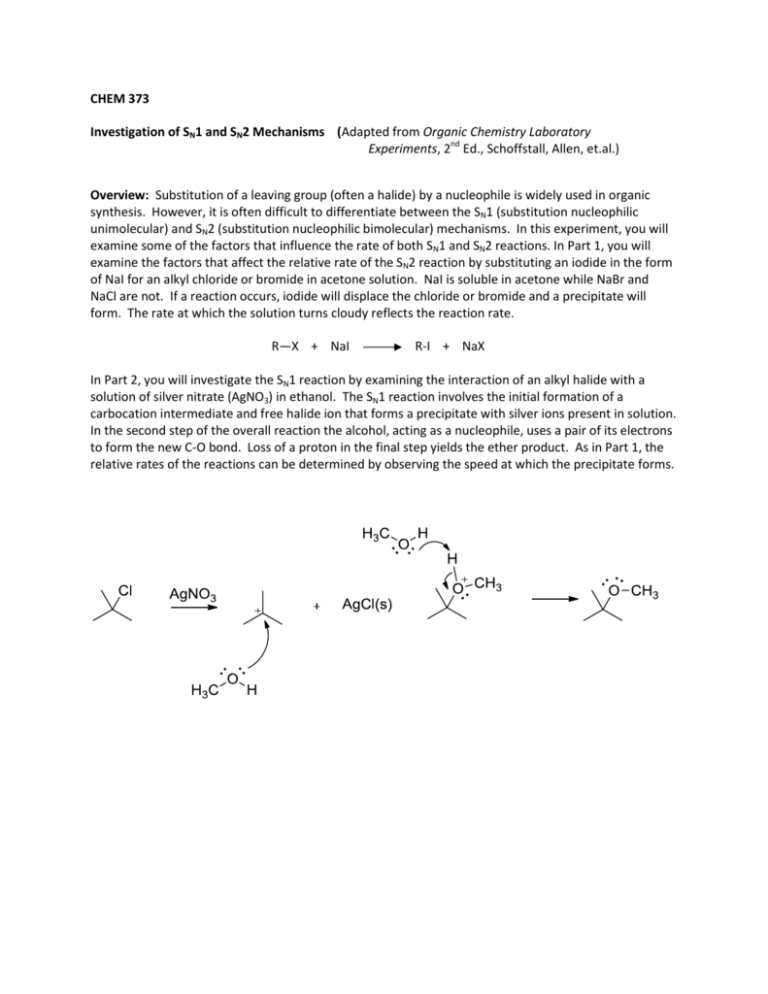
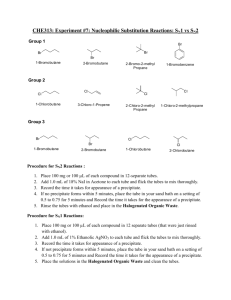
CHEM 373 Investigation of SN1 and SN2 Mechanisms (Adapted from Organic Chemistry Laboratory Experiments, 2nd Ed., Schoffstall, Allen, et.al.) Overview: Substitution of a leaving group (often a halide) by a nucleophile is widely used in organic synthesis. However, it is often difficult to differentiate between the SN1 (substitution nucleophilic unimolecular) and SN2 (substitution nucleophilic bimolecular) mechanisms. In this experiment, you will examine some of the factors that influence the rate of both SN1 and SN2 reactions. In Part 1, you will examine the factors that affect the relative rate of the SN2 reaction by substituting an iodide in the form of NaI for an alkyl chloride or bromide in acetone solution. NaI is soluble in acetone while NaBr and NaCl are not. If a reaction occurs, iodide will displace the chloride or bromide and a precipitate will form. The rate at which the solution turns cloudy reflects the reaction rate. R—X + NaI R-I + NaX In Part 2, you will investigate the SN1 reaction by examining the interaction of an alkyl halide with a solution of silver nitrate (AgNO3) in ethanol. The SN1 reaction involves the initial formation of a carbocation intermediate and free halide ion that forms a precipitate with silver ions present in solution. In the second step of the overall reaction the alcohol, acting as a nucleophile, uses a pair of its electrons to form the new C-O bond. Loss of a proton in the final step yields the ether product. As in Part 1, the relative rates of the reactions can be determined by observing the speed at which the precipitate forms. Remember that all data and observations should be entered directly into the notebook. Do not use the Report Form to record data while the experiments are in progress, it should be filled out only after the experiment is completed. Reading Assignment: Organic Chemistry, 3rd Ed., Smith, J., Chap.7 – pp. 262-266. Part 1: the SN2 reaction Effect of the alkyl halide structure Into each of three clean, dry 10 mL test tubes or vials add 2 mL of a 15% solution of sodium iodide in acetone. Next, add 2 drops of 1-bromobutane to tube one, 2 drops of 2-bromobutane to tube 2, and 2 drops of 2-bromo-2-methyl propane to tube 3. Stopper the tubes with corks or caps if using vials and shake to mix. Observe during the first 15 minutes and then periodically throughout the rest of the lab period. Record your observations. Steric effects Into each of three clean, dry 10 mL test tubes or vials add 1 mL of a 15% solution of sodium iodide in acetone. Next, add 2 drops of 1-bromobutane to tube one, 2 drops of 1-bromo-2-methylpropane to tube 2, and 2 drops of 1-bromo-2,2-dimethyl propane to tube 3. Stopper the tubes with corks or caps if using vials and shake to mix. Record your observations. Effect of the leaving group Into each of two clean, dry 10 mL test tubes or vials add 1 mL of a 15% solution of sodium iodide in acetone. Next, add 2 drops of 1-bromobutane to tube one, and 2 drops of 1-chlorobutane to tube 2. Stopper the tubes with corks or caps if using vials and shake to mix. Record your observations. Determination of the rate law Measure 1.0 mL of a 15% solution of sodium iodine in acetone into two clean, dry test tubes. At the same time (or do one at a time, marking the time carefully!), add 0.1 mL (or 2 drops) of 1.0 M 1bromobutane to one test tube and add 0.1 mL (or 2 drops) of 2.0 M 1-bromobutane to the other. Record your observations and compare the rates of the two reactions. Measure 1.0 mL of a 1.0 M solution of 1- bromobutane into two clean, dry test tubes. Into one test tube, add 0.1 mL (or 2 drops) of a 7.5% solution of sodium iodide in acetone. At the same time add 0.1 mL (or 2 drops) of 15% solution of sodium iodide in the acetone into the other test tube. (Alternately, use one test tube at a time, noting carefully the amount of time for precipitate formation.) Record your observation and compare the rates of the two reactions. Part 2: the SN1 reaction Effect of the alkyl halide structure Into each of three clean, dry 10 mL test tubes or vials add 2 mL of a 0.1 M solution of silver nitrate in absolute ethanol. Next, add 1 drop of 1-bromobutane to tube one, 1 drop of 2-bromobutane to tube 2, and 1 drop of 2-bromo-2-methyl propane to tube 3. Stopper the tubes with corks or caps if using vials and shake continuously. Watch closely for evidence of the cloudiness or the formation of a white precipitate. Effect of the leaving group Measure 2 mL of a 0.1 M solution of silver nitrate in the absolute ethanol into two clean, dry test tubes. Add 1 drop of 2-bromo-2-methylpropane into one test tube. At the same time, add 1 drop of 2-chloro-2methylpropane into the other test tube. Stopper and shake. Record your observations. Effects of Solvent Polarity Measure 2 mL of a 0.1 M solution of silver nitrate in absolute ethanol into a dry test tube. Into the second dry test tube, measure 2 mL of 0.1 M silver nitrate solution in 5% ethanol/95% acetone. At the very same time (get a partner to help!) add 1 drop of 2-chloro-2-methylpropane to each test tubes. Stopper and shake. Observe. Record your observations. Determination of the Rate Law To a clean, dry test tube, add 0.5 mL of 0.1 M 2-chloro-2-methylpropane in ethanol. To another clean, dry test tube, 0.5 mL of 0.2 M 2-chloro-2-methylpropane in ethanol. At the same time, add 1.0 mL of 0.1 M silver nitrate solution in ethanol to each of the two test tubes. (Alternately, do one at a time and carefully note the time it takes for precipitation.) Record your observations and compare the rates for the two reactions. To a clean, dry test tube, add 1.0 mL of 0.1 M silver nitrate to ethanol. To another clean, dry test tube, add 0.5 mL of 0.1 M silver nitrate solution in absolute ethanol and 0.5 mL absolute ethanol (to ensure that the volumes are the same in the two test tubes). At the same time, add 1.0 mL of 0.1 M 2chloro-2-methylpropane in ethanol to each of the two test tubes. (Alternately, work with one test tube at a time and carefully note the time it takes for precipitation.) Record your observations and compare the rates if the two reaction. Pre-Lab Assignment Investigation of SN1 and SN2 Mechanisms Name_____________________________________ 1. Draw skeletal structures for all of the alkyl halides you will be using and label them as primary, secondary, or tertiary halides. 2. A student wishes to determine the rate law for the reaction A + B gives C. The student doubles the concentration of A while holding the concentration of B constant and finds that the rate of the reaction doubles. The student then doubles the concentration of B while holding the concentration of A constant and finds that the rate is unchanged. Write the rate law for this reaction.