File - Grade 10 Student's hub
advertisement

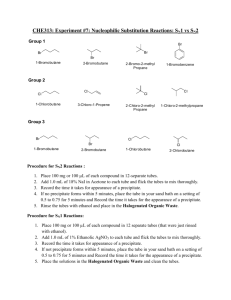
Determination of contents of cold drinks Muhammad Hassan Sohail Grade: 10 B3 Theory Cold drinks of different brands are composed of alcohol, carbohydrates, carbon dioxide, phosphate ions etc. These soft drinks give feeling of warmth, lightness and have a tangy taste which is liked by everyone. Cold drinks detected by the PH paper are bit acidic in nature. The pH values also depend upon the acidic contents such as citric acid and phosphoric acid. Carbondioxide present in cold drinks can be estimated by presence of effervescence on shaking the bottle. Actually Carbondioxide is filled in bottle under pressure and is present in the drink as bicarbonates which is a weak acid. That’s why drinks are sour in taste. Apparatus used i. ii. iii. iv. v. Test Tube Test Tube Holder Test Tube Stand Stop Watch Beaker vi. vii. viii. ix. x. xi. Burner pH Paper Tripod Stand China Dish Wire Gauge Water Bath Chemicals needed i. ii. iii. iv. v. vi. vii. viii. Iodine Solution Potassium Iodine Sodium Hydroxide Fehling’s A & B Solution Lime Water Concentrated HNO3 Benedict Solution Ammonium Molybdate Test for PH Procedure: Small samples of cold drinks of different brands were taken in a test tube and blue litmus paper was put inside each test tube. Observation Number 1 2 3 4 Name of the drink Coca-cola Sprite Lemonade Fanta Change Blue litmus paper turns red Blue litmus paper turns red Blue litmus paper turns red Blue litmus paper turns red Test for carbon dioxide Procedure: Bottles of fizzy drinks were opened and put in a test tube. Then limewater was put in the test tube and the product was observed. Observation Number 1 2 3 4 Name of the drink Coca-cola Sprite Lemonade Fanta Time taken 26.5s 21s 35s 36s Product formed Co2 is present Co2 is present Co2 is present Co2 is present Chemical reaction involved Ca(OH)2(s) + CO2(g) CaCO3(s) + H2O(s) Test for glucose Procedure: Glucose is a reducing sugar acid. Its presence is detected by the Benedict’s regent test:Small samples of cold drinks of different brands were taken in a test tube and a few drops of Benedict’s reagent were added. The test tube was heated for few seconds. Formation of reddish colour confirmed the presence of glucose in cold drinks. Observation Number 1 2 3 4 Name of the drink Coca-cola Sprite Lemonade Fanta Product formed Reddish colour precipitate Reddish colour precipitate Reddish colour precipitate Reddish colour precipitate Test for phosphate Procedure: Small samples of cold drinks were taken in separate test tubes and Ammonium Molybdate with concentrated Nitric was added to it. The solution was heated. Appearance of yellow precipitate confirmed the presence of phosphate in cold drinks. Observation Number 1 2 3 4 Name of the cold drink Coca-cola Sprite Lemonade Fanta Product formed Yellow precipitate Yellow precipitate Yellow precipitate Yellow precipitate Chemical reaction involved: NaHPO4 + 12(NH4)2MoO4 + 21HNO3 + 3H+ (NH4)3PO4.12MoO3 + 21HN4NO3 + 12H2O Test for alcohol Procedure: Small samples of cold drinks were taken in separate test tubes and Iodine with Potassium Iodide and Sodium Hydroxide (NaOH) solution was added to each test tube. Then the test tubes were heated in hot water bath for 30 minutes. Appearance of yellow colored precipitate tells us that alcohol is present the presence of alcohol in cold drinks. Observation Number 1 2 3 4 Name of the cold drink Coca-cola Sprite Lemonade Fanta Product formed Yellow precipitate Yellow precipitate Yellow precipitate Yellow precipitate Chemical reaction involved: CH3CH2OH + 4I2 + 6NaOH CHI3 + HCOONa + 5NaI + 5H2O Conclusion All cold drinks contain glucose, alcohol, phosphate and carbon dioxide. All cold drinks are acidic in nature. By looking at the tables, we see that Coca Cola is the most acidic and Limca is least acidic in between Fanta and Sprite. We also concluded that amoung the cold drinks tested, Sprite has the maximum amount of CO2.