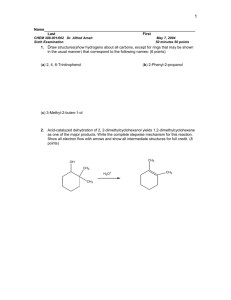
CH3CH2CCH(CH3)2 O CH2CH2CHO
advertisement

Chem 226 — Problem Set #9 — “Fundamentals of Organic Chemistry,” 4th edition, John McMurry. Chapter 9 2. Name the following aldehydes and ketones. (a) O (b) CH2CH2CHO CH3CH2CCH(CH3)2 H CH3 O (c) O CH3CCH2 CH2 CH2CCH2CH3 (d) H C O H (a) 2-methyl-3-pentanone, (b) 3-phenylpropanal, (c) 2,6-octanedione, (d) (1R,2R)-2-methylcyclohexanecarbaldehyde 4. How could you prepare pentanal from the following starting materials? (a) 1-pentanol, (b) CH3CH2CH2CH2COOH, (c) 5-decene (a) CH3CH2CH2 CH2 CH2OH PCC CH2Cl2 CH3CH2CH2CH2 CHO O CH3CH2CH2 CH2C OH (b) CH3CH2CH2 CH2CH2OH 1. LiAlH4 + 2. H3O PCC (c) CH3CH2 CH2 CH2C OH CH3CH2 CH2 CH2CH2OH CH3CH2CH2 CH2 CHO CH2Cl2 CH3CH2 CH2 CH2CH CHCH2CH2CH2 CH3 O CH3CH2CH2CH2 CH2 OH 1. LiAlH4 + 2. H3 O PCC CH2Cl2 KMnO4 H3O + CH3CH2CH2CH2CH2 OH CH3CH2CH2 CH2 CHO 5. How could you prepare 2-hexanone from the following starting materials? (a) 2-hexanol, (b) 1-hexyne, (c) 2-methyl-1-hexene OH O (a) CH3 CH2CH2CH2CHCH3 K 2CrO7 CH3CH2CH2CH2CCH3 O (b) CH3 CH2CH2CH2C CH CH3 (c) CH3 CH2CH2CH2C CH2 H2SO 4 HgSO4 H2O CH3CH2CH2CH2CCH3 O KMnO 4 H3O CH3CH2CH2CH2CCH3 + (b) The enol that initially forms here undergoes keto-enol tautomerism. Terminal alkynes give methyl ketones; they follow Markovnikov’s rule. (c) Potassium permanganate under basic conditions would hydroxylate the alkene to form a vicinal diol, but under acidic conditions it completely cleaves the double bond. The =CH2 group would become carbon dioxide. 6. How would you carry out the following transformations? More than one step may be required. (a) 3-hexene 3-hexanone (b) benzene 1-phenylethanol H (a) CH3CH2CH CHCH2CH3 H2O CH3CH2CH CHCH2CH3 H2 SO 4 H K 2Cr2 O7 CH3CCl AlCl3 NaBH4 O CH3CH2CH CCH2CH3 O (b) OH C CH3 O H C CH3 OH 9. The reduction of a ketone to a secondary alcohol on treatment with NaBH4 is a nucleophilic addition reaction in which the nucleophile hydride ion (:H-) adds to the carbonyl group, and the alkoxide ion intermediate is then protonated. Show the mechanism of this reduction. H H B H H C H H C C O O H O H O CH2CH3 O CH2CH3 Ethanol is frequently used as a solvent in these reactions. In any event a proton must be supplied by something in the solution. 11. When dissolved in water, trichloroacetaldehyde (chloral, CCl3CHO) exists primarily as the gem diol, chloral hydrate. Show the structure of chloral hydrate. CCl3CH(OH)2 14. Show the mechanism of the acid-catalyzed formation of a cyclic acetal from ethylene glycol and acetone. H2 C CH2 H2C OH OH H3 C C CH3 H3 C O C CH3 H3C O H CH2 H2C CH2 OH OH O OH C CH3 H3C C O H O H H H+ H2C CH2 O H3C O C CH3 H3 C CH3 O H C CH3 H2C CH2 H2C CH2 O OH O OH H3C C CH3 H3 C O C H3C CH3 C CH3 + H2O 17. Show the products obtained from addition of CH3MgBr to the following compounds. (a) cyclopentanone, (b) benzophenone, (c) 3-hexanone One assumes that McMurry means the alcohol one would get upon acidification after adding the Grignard reagent to the ketone. HO C (b) (a) OH H3 C CH3 OH (c) CH3CH2CCH2 CH2 CH3 CH3 18. How might you use a Grignard addition reaction to prepare the following alcohols? (a) 2-methyl-2-propanol, (b) 1-methylcyclohexanol, (c) 3-methyl-3-pentanol To make a tertiary alcohol, we can react a ketone with the Grignard reagent. To make a secondary alcohol we can react an aldehyde with the Grignard reagent. To make a primary alcohol we can react formaldehyde with the Grignard reagent. OH CH3CCH3 CH3 H3C 1. CH3MgBr + 2. H3O O CH3CCH3 O OH 1. CH3MgBr + 2. H3O OH CH3CH2 CCH2CH3 CH3 1. CH3MgBr + 2. H3O O CH3CH2CCH2 CH3 19. Identify the different kinds of carbonyl groups in the following molecules. carboxylic acid HO C O (a) ester (carboxylic) O CH3 ester N COCH3 OCCH3 (b) OCC 6H5 H O H ester O ester (cyclic CH2OH esters are sometimes called lactones) HOHC O O (c) O OH ketone We usually call the esters of carboxylic acids just esters since this type of ester is so common. However, other acids – sulfonic acids, for example – can also form esters. 22. Draw and name the seven aldehydes and ketones with the formula C5H10O. O O CH3CH2CH2CH2CH pentanal CH3 O CH3CHCH2CH 3-methylbutanal 23. O CH3 CH2CH2CCH3 2-pentanone CH3 CH3 CHCCH3 CH3 CH2CCH2CH3 3-pentanone CH3 HCCHCH2CH3 CH3 HCCCH3 O O O CH3 3-methyl-2-butanone 2-methylbutanal 2,2-dimethylpropanal Which of the compounds you identified in question 22 are chiral? Our approach here will be to look for stereocenters. If a molecule has one, and only one stereocenter, it will be chiral. If a molecule does not have any stereocenters it is unlikely to be chiral. [ In this course it is very unlikely we will see chiral molecules that do not have stereocenters.] If a molecule has two or more stereocenters it will be chiral unless it is a meso structure. 2-Methylbutanal is the only molecule above that has has a stereocenter, and it has just one (C-2). It is chiral. 32. Starting from 2-cyclohexenone and any other reagents needed, how would you prepare the following substances? (More than one step may be required.) (a) 1,3-cyclohexadiene, (b) 1-methylcyclohexanol, (c) cyclohexanol, (d) 1-phenyl-2-cyclohexen-1-ol (a) OH H2SO 4 heat OH H3C 1. CH3MgBr O NaBH4 2. H3O O (b) OH H2 Pt + NaBH4 MgBr (c) + H3O 34. (d) OH Use a Grignard reaction on an aldehyde or ketone to synthesize the following compounds. (a) 2-pentanol, (b) 2-phenyl-2-butanol, (c) 1-ethylcyclohexanol, (d) diphenylmethanol When undertaking a Grignard synthesis of an alcohol keep in mind that two of the groups (including all hydrogens) attached to the carbon bearing the OH group will come from the carbonyl compound and one of them (not a hydrogen) will come from the Grignard reagent. OH H3C C CH2 CH2 CH3 H + H3 O O H3O + O H3C C C CH2CH2 CH3 + H CH3MgBr + H BrMgCH2CH2 CH3 (a) Viewed one way, we are trying to make a secondary alcohol, so we employ an aldehyde and a Grignard reagent. The Grignard reagent can provide either of the boxed alkyl groups, while the aldehyde will provide the other. The hydrogen must be provided by the aldehyde – there is no HMgX that can react with a ketone to give a secondary alcohol. (b) Since we are trying to make a tertiary alcohol, we will employ a ketone and a CH3CCH2CH3 CH3C + BrMgCH2CH3 Grignard reagent. The ketone will provide two of the groups attached to the carbon H3O+ holding the OH (as well as that carbon itself) and the Grignard reagent will provide the third group. In this case I have arranged for the Grignard to provide the boxed ethyl group and the acetophenone will provide the methyl and phenyl groups. Two other combinations could have been used. What are they? OH O (c) We are, again, trying to make a + BrMgCH2CH3 tertiary alcohol, so we will use a ketone and the Grignard. The carbon holding the OH and two of the groups attached + H3O to it must come from the ketone and the third group must come from the Grignard. The situation here is made a little interesting by virtue of the ring, since the ring constitutes two of the groups attached to the OH bearing carbon, but is “one piece” and cannot come from two different sources. Consequently, the only choice for the ketone is cyclohexanone and this forces the Grignard to be ethylmagnesium halide. HO CH2CH3 O H O C OH C H + H3O + MgBr (d) A secondary alcohol requires an aldehyde and Grignard reagent. There is no choice here since two of the groups are phenyls. The aldehyde must be benzaldehyde and the Grignard must provide another phenyl group.