Matter
Learning Targets
Name:__________________________
Each of the following “Learning Targets” below are ideas or skills you need to know or do to be able to pass the unit. Circle either “Yes,” “Need practice,” or
“No” for each learning target. If you didn’t circle “Yes”, look over the “In-Class Resources” and the “Additional Resources” for additional help.
YES = I understand or can do it
Circle
One
↓
Yes
Need
practice
No
Yes
Need
practice
No
Yes
Need
practice
No
Yes
Need
practice
Need Practice = I kind of get it
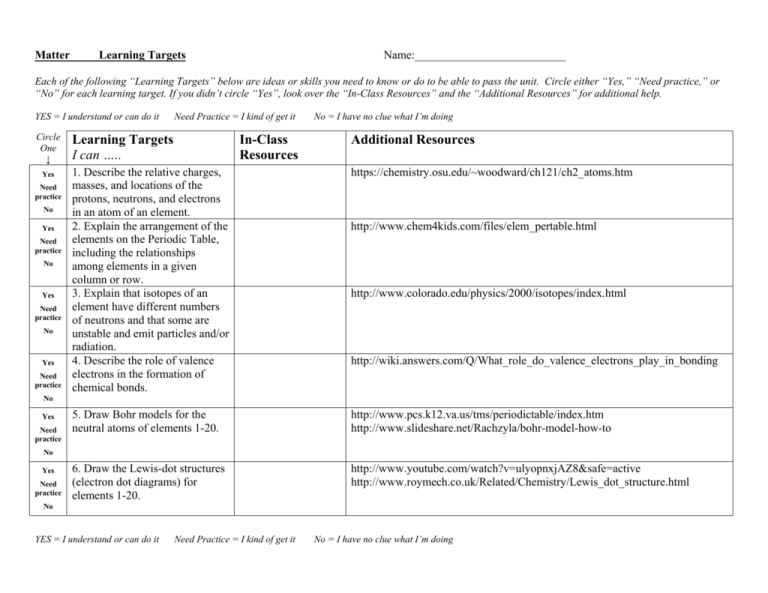
Learning Targets
I can …..
In-Class
Resources
1. Describe the relative charges,
masses, and locations of the
protons, neutrons, and electrons
in an atom of an element.
2. Explain the arrangement of the
elements on the Periodic Table,
including the relationships
among elements in a given
column or row.
3. Explain that isotopes of an
element have different numbers
of neutrons and that some are
unstable and emit particles and/or
radiation.
4. Describe the role of valence
electrons in the formation of
chemical bonds.
No = I have no clue what I’m doing
Additional Resources
https://chemistry.osu.edu/~woodward/ch121/ch2_atoms.htm
http://www.chem4kids.com/files/elem_pertable.html
http://www.colorado.edu/physics/2000/isotopes/index.html
http://wiki.answers.com/Q/What_role_do_valence_electrons_play_in_bonding
No
Yes
Need
practice
5. Draw Bohr models for the
neutral atoms of elements 1-20.
http://www.pcs.k12.va.us/tms/periodictable/index.htm
http://www.slideshare.net/Rachzyla/bohr-model-how-to
6. Draw the Lewis-dot structures
(electron dot diagrams) for
elements 1-20.
http://www.youtube.com/watch?v=ulyopnxjAZ8&safe=active
http://www.roymech.co.uk/Related/Chemistry/Lewis_dot_structure.html
No
Yes
Need
practice
No
YES = I understand or can do it
Need Practice = I kind of get it
No = I have no clue what I’m doing
YES = I understand or can do it
Circle
One
↓
Yes
Need
practice
No
Yes
Need
practice
No
Yes
Need
practice
No
Need Practice = I kind of get it
Learning Targets
In-Class
Resources
7. Use the Periodic Table to
determine the number of protons,
electrons and neutrons in an atom
of a given element.
8. Use the Periodic Table to
determine the number of energy
levels and valence electrons in an
atom of a given element.
9. Describe trends across rows
and down columns in the
Periodic Table
YES = I understand or can do it
Need Practice = I kind of get it
No = I have no clue what I’m doing
Additional Resources
http://www.college-cram.com/study/chemistry/atoms-and-molecules/using-theperiodic-table/
http://www.youtube.com/watch?v=wGz7uqMxddQ&safe=active
http://www.college-cram.com/study/chemistry/atoms-and-molecules/using-theperiodic-table/
http://www.youtube.com/watch?v=wGz7uqMxddQ&safe=active
http://chemistry.about.com/od/periodictableelements/a/periodictrends.htm
http://www.chem.tamu.edu/class/majors/tutorialnotefiles/trends.htm
No = I have no clue what I’m doing
o
l' \ ~COIVMI')S
The Periodic Table of the Elements
\
~4--3 -;)
+)
\
~
3
Lithium
Beryllium
6.941
9.012182
11
Na
12
Mg
Sodium
Magnesium
22.989770
20
Ca
5
W
21
Sc
31
Ga
26
Fe
32
Ge
Calcium
Zinc
Gallium
65.39
69.723
72.61
48
49
In
50
Sn
Rb
Strontium
Cd
Molybdenum
85.4678
95.94
102.90550
55
Cs
74
W
77
78
Ir
Pt
.
132.90545
59
Pr
-teN \0 ids
81
TI
82
Pb
83
Bi
lllallillIn
Lead
207.2
108
Hs
112
113
114
65
Tb
66
Dy
67
Ho
Terbium
Dysprosium
61
Iridium
Pm
62
Sm
Promelhill111
Samarium
144.24
(145)
150.36
92
93
Np
94
63
..•.
Yb
71
Lu
Ytterbium
Lutetium
68
Er
Tm
El'bium
Thulium
164.93032
167.26
173.04
8
99
100
Pu
Cf
Es
Fm
102
No
244)
251)
(252
Liquid S ~f
.••..
1
Holmium
Eu
16250
Uranium
231.03588
1995 IUPAC masses and Approved Names from htlp:l/ww'\\'.chclll gmw,ac.ukiillpilc/AtWt!
masses for 107-111 from C&EN. March) 3, 1995. p. 35
112 from hllQ:i!ww\-v.<'si.dehI12c.hul1l
121.760
204.3833
U
Protactinium
Antimony
Mercury
60
Nd
NeodYlllill1n
Tin
118.710
200.59
1
0:: me.+o.. \ S
: nOn- me.to..ls
Indium
114.818
190..23
Osmium
Cesium
~ N\ e
5
26.981538
23
V
40.078
Rubidium
R
o
Potassiurrt
38
Sr
~
W(iaht:-
.
39.0983
37
5
A~mit. ~Mbo\
Atomi C.rtlA$s /
24.3050
19
K
4
4
AtOMi(. ~
4
Be
~,
~
rOOM
mOSt: r e ~ <...1 i\J -e..
pe.rQ.. tvv' e
e le.rne.)'\ \-$
t-e.rY'
Atomic
#
Element
Column/Group/Family
Radius
Name
Names
A\ ~~\\ne Me:\-cd$ 5. Nd-ro~~ni;..m,I"12: Mko.\ir\t Eo.f"'f-h M~to-ls 6.~~e.n
~o.rnil~
3: Boron Fc,..mi l~__
7. "Q..\Q%o..=O\S~
_
4: Co.rb"n ~OlY\i I~
8. Noble 1iCl.S es
3
Lithium
152
4
Beryllium
111
5
Boron
86
6
Carbon
77
7
Nitrogen
70
8
Oxygen
73
9
Fluorine
72
Particle
Charge
Mass
Location
10
Neon
71
Proton
-+-\
Nvc...\ e.uS
11
Sodium
186
Neutron
I ~t"\U
\ Pt.MV
12
Magnesium
160
13
Aluminum
143
14
Silicon
118
15
Phosphorus
108
16
Sulfur
106
17
Chlorine
99
18
Argon
97
19
Potassium
232
20
Calcium
197
1:
0
... \
Electron
'4'00 AH\j
of O~ v
An ion is a
Lt\le\~
v
= the o.tomlC nvrnber
The number of electrons = t be 0..-\-omi c.. Y\ v mbet"'
The number of neutrons =
RO\Jnc.\e& o..t()mit mCLSS - o.:h;>mi<.. rwmber
The atomic number, or the number of
are elements that have the same number of
~y-()n
tf\er~1.(
The number of protons
tells you the identity
Isotopes
euS
\,1\.><" \
~r()\-ohS
of an atom.
VV'" C) fO\,\$
,but different
numbers
S.
cho.r~eO
atom. Ions become charged by gaining or losing
e\e,c...-n-O't\S
Columns
Columns go
~p c..nQ Qc:>wn
on the Periodic Table.
and ~m\
Columns are also called ~
\\t..S.
The column number tells you the number of \/<:A \
Valence electrons
are the electrons
in the
en<.e.
O\ltS \ d.~
electrons.
energy level.
Rows
Rows go hOY"\"2.onto..\l\.j- on the Periodic Table.
Rows are also called
¥e.~\ods
Rows tell you the number of t,Y\trC!l,C
.
le:.\lt-ls
Alpha Decay:
_
Beta Decay:
_
Gamma Decay:
_
Alpha Decay Example:
_
Beta Decay Example:
_
Science Spectrum Answer Key continued
6. In both cases, the variables are directly
7.
8.
9.
10.
11.
7. Charged particles move in response to a
related. As one changes, the other changes
in the same direction.
pressure and amount of gas
Direct; as one variable increases, the other
variable increases.
Yes; a straight-line graph shows a
proportional relationship.
Boyle’s law
Inverse; as one variable increases, the other
decreases.
8.
9.
10.
11.
Review
1. Rutherford’s experiment showed that the
Review
1. The particles in a gas bump each other
2.
3.
4.
5.
and the walls of the container, producing
pressure.
Gay-Lussac’s Law; as temperature increases,
pressure increases. In summer, the
temperature of the air inside the tires
would be higher than in the winter and
pressure would increase.
The temperature of the particles in the balloon
would increase. When temperature increases,
the volume of the gas will increase and the
balloon will increase in volume and may
break. Charles’s law predicts this result.
Temperature and pressure are inversely
related. As one variable increases, the other
decreases.
Students should draw a graph with
temperature on the x-axis and pressure
on the y-axis. They should draw a straight
line from the origin to the top right corner.
Possible title: “Pressure Versus Temperature
for a Gas at a Constant Volume”
2.
3.
4.
5.
6.
7.
1. protons and neutrons
2. The mass of an electron is much smaller
than the mass of a proton.
3. two
4. They have the same number of protons as
SECTION 1 THE DEVELOPMENT OF
ATOMIC THEORY
1. the Greek word atomos OR a Greek word
4.
5.
6.
positive charge in an atom is concentrated
in a nucleus at its center.
Possible answer: The law of definite proportions states that the relative masses of
elements in a compound are always the same.
Both Dalton and Democritus thought that an
atom was a tiny, indivisible particle.
In Thomson’s atomic theory, electrons were
embedded in a mass of positive charge.
In Rutherford’s atomic theory, electrons
orbited a dense, positively charged nucleus.
He concluded that the particles came
from atoms in the cathode and that the
particles were the same in atoms of
different elements.
There are 164 g of nitrogen in 200 g of
ammonia.
There are 27 g of hydrogen in 150 g of
ammonia.
SECTION 2 THE STRUCTURE OF ATOMS
Chapter 4 Atoms
2.
3.
magnet, but light rays do not.
In Thomson’s model, the atom contains
smaller particles. In Dalton’s model, the
atom was indivisible.
They would either pass straight through or
be deflected slightly.
He repeated the experiment.
Student should label the nucleus “positive”
and the electrons “negative.”
5.
6.
7.
8.
9.
10.
11.
12.
13.
meaning “unable to be cut or divided”
There was no evidence to support it.
Dalton had scientific evidence to support his
theory.
24 g
New observations did not support Dalton’s
theory.
the anode
electrons.
the electric force
It has 13 protons and 13 electrons.
They can have different numbers of neutrons.
one
atomic number8; mass number 16
17
18
the unified atomic mass unit
35.453 u
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Interactive Reader
5
Answer Key
Science Spectrum Answer Key continued
14. the basic unit used to measure the amount
15.
16.
17.
18.
13. Student should label the image with both
of a substance
29 g
24.3 g/mol
molar mass of Cu63.55 g/mol;
(3.20 mol Cu) × (63.55 g Cu/mol Cu)
203 g Cu
molar mass of C12.01 g/mol; molar mass
of H 1.01 g/mol; molar mass of
CH4 (12.01 g/mol) + (4) × (1.01 g/mol)
16.05 g/mol
electrons in one energy level “ground
state” and should label the other image
“excited state”
Review
1. s or p
2. Energy levels contain orbitals.
3. The first energy level contains two
4.
Review
1. Mass number is the number of protons and
2.
3.
4.
5.
neutrons in the nucleus of an atom. Atomic
mass is the mass of a single atom in grams
or in unified atomic mass units.
top row: 7
second row: 7
third row: 27
fourth row: 37
bottom row: 87
Atoms A and B have the same atomic
number but different mass numbers, so
they are isotopes.
Atom D contains 37 protons. Since atoms are
neutral, it must also contain 37 electrons.
molar mass of glucose 180.2 g/mol;
(300 g glucose)(180.2 g/mol) 1.66 mol
5.
6.
7.
Chapter 5 The Periodic
Table
SECTION 1 ORGANIZING THE ELEMENTS
1. atomic mass
2. Scientists had not yet discovered elements
SECTION 3 MODERN ATOMIC THEORY
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
12.
electrons. The second energy level
contains the other five electrons.
An atom of nitrogen has two electrons in the
first energy level and five in the second. The
second energy level is the outermost energy
level, so nitrogen has five valence electrons.
We can’t know an electron’s exact location,
speed, or direction in an atom.
The electrons in an aluminum atom are located
in the first, second, and third energy levels.
It has two electrons in the first energy level,
eight in the second energy level, and three
in the third energy level. The third energy
level is the outermost energy level, so
aluminum has three valence electrons.
with the properties that fit the pattern.
Mendeleev predicted that scientists would
find those elements.
3. Te and I did not fit the pattern of chemical properties unless they were put in the
wrong order of atomic mass.
4. atomic number
5. The group it is in; elements in a group have
similar chemical properties. Properties of
elements change across a period.
It must gain energy.
lost
Bohr’s model worked only for hydrogen.
a region in an atom in which an electron is
most likely to be
Student should label the large shaded region
around the nucleus “orbital.”
In both models, electrons can be located
only in certain energy levels.
the first and second
electrons in the outer energy level of an atom
two
They lie along different directions in space.
10
the lowest state of energy of the electron
Review
1. The chemical properties of oxygen are more
similar to those of sulfur because they are
members of the same group. Elements in
the same group share similar properties.
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Interactive Reader
6
Answer Key
The Periodic Table Journey
Today you are going to go on a journey of discovery about the periodic table. To make
it even more realistic, you are actually going to travel on a journey. The information
that you need is placed around the school and you will travel to find it. To make this
work we have to have some ground rules, so here goes:
1. You must stay with your group.
2. Go in order – the locations are listed for each section, when you finish one go
to the next one down the list.
3. Only one group at a spot at a time.
4. You may not bother any other classes.
5. You may not be loud in the hallways.
Please write all of your answers in this packet and then place this in your 3-ring
binder. Have a pleasant journey and don’t forget to write!
Transition Metals (Door W1)
1. Where do you find the transition metals on the Periodic Table? (If you haven’t
been to the “The Periodic Table” spot or through the “Label Loop”, you won’t be
able to answer this question yet.)
They are found in the middle of the Periodic Table (the shorter columns).
2. List 5 properties that transition metals have.
A.
B.
C.
D.
E.
3. What are the only magnetic transition metals?
4. List some special properties of the following transition metals:
Platinum:
Nickel:
Silver:
Iron:
Nonmetals (Door W2)
1. Where do you find the nonmetals on the Periodic Table? (If you haven’t been
to the “The Periodic Table” spot or through the “Label Loop”, you won’t be able
to answer this question yet.)
On the right side of the Periodic Table
2. What nonmetals are solids at room temperature?
3. What nonmetal is a liquid at room temperature?
Bromine
4. List some special properties of the following nonmetals:
Hydrogen:
Helium:
Carbon:
5. What nonmetals are present in the air that you breathe?
Isotopes (Door W3)
1. What is the definition of an isotope?
Isotopes are atoms with the same number of protons and different numbers of
neutrons.
2. What is the “Nucleon Number”?
The nucleon number is the sum of the protons and the neutrons.
3. What is the name that we have been using for the nucleon number?
Atomic Mass
4. What two isotopes are listed on the poster?
Carbon – 12 and Carbon - 14
5. What is the difference between the two isotopes from the question #4?
Carbon – 14 has two more neutrons than Carbon – 12.
6. What is the same for the two isotopes from question #4?
Both isotopes have the same number of protons.
What is an Element? (Door E4)
1. What is the definition of an element?
2. How many of the known elements are naturally occurring?
3. What are allotropes? Give an example.
Allotropes are different forms of the same element. Coal, graphite and
diamond are all allotropes of carbon.
4. What are the two most abundant elements in the Universe?
Hydrogen and Helium
5. What are the five most abundant elements in your body? What percent of your
body do they make up?
6. What are the two rarest elements on earth?
How to Read the Periodic Table (Door E3)
1. What does the atomic number tell us about an element?
The atomic number tells you the number of protons and electrons in an atom.
2. What does the atomic weight (mass) tell us about an element?
The atomic weight (mass) is the sum of the protons and neutrons in an atom.
3. What do the rows on the Periodic Table tell us?
The row number tells you the number of energy levels (rings of electrons) that
an atom has.
4. What do the columns on the Periodic Table tell us?
The columns tell you the number of valence electrons an atom has.
5. How do you find the number of neutrons that an atom of an element has?
# of neutrons = atomic mass – atomic number
Metals (Main Office Door)
1. Where do you find the metals on the Periodic Table? (If you haven’t been to
the “The Periodic Table” spot or through the “Label Loop”, you won’t be able to
answer this question yet.)
You find the metals on the left side of the Periodic Table.
2. List six common properties of metals:
A.
B.
C.
D.
E.
F.
3. What are alloys and why are they so useful?
4. What are ores?
5. Why are metalloids?
Metalloids are elements that have properties of both metals and non-metals.
The Periodic Table (Door E2)
1. Who drew up the first periodic table? When did he do this?
Dmitri Mendeleev
2. What are the columns called?
Groups or Families
3. What are the rows called?
Periods
4. What happens to the size of the atoms as you go across a row (period)?
5. What happens to the size of the atoms as you go down a column (group)?
6. How can we use the Periodic Table to make predictions about elements and
compounds?
Active Metals (By Our Fire Exit Door)
1. Where do you find the active metals on the Periodic Table?
2. Why do we call them active metals?
We call them active metals because they are very reactive.
3. What do all of the Alkali Metals have in common?
4. What do all of the Alkali-Earth Metals have in common?
5. Which two active metals are radioactive?
6. What is one of the earth’s most abundant metals? Where do you find it in you?
Calcium is one of the most abundant metals on Earth. You have calcium in your
bones.
7. What active metals do our bodies need small amounts of?
Sodium and Potassium
The Label Loop
Starting at A207 (that room sounds familiar) go around the loop and label the things
on your Periodic Table. You will need an assortment of colored pencils for your group
(eight should do it). When you get back to A207, you are done with this station.
Protons, Neutrons, and Electrons Practice Worksheet
Element
Name
Atomic
symbol
Atomic
Mass
(rounded)
Atomic
number
Protons
Neutrons
Electrons
Valence
Electrons
Boron
B
11
5
5
6
5
3
Metal,
Metalloid
or Non metal
Metalloid
Sodium
Na
24
11
11
13
11
1
Metal
Gallium
Ga
68
31
31
37
31
3
Metal
Yttrium
Y
89
39
39
50
39
3
Metal
Copper
Cu
64
29
29
35
29
1
Metal
Technetium
Tc
98
43
43
55
43
7
Metal
Lead
Pb
207
82
82
125
82
6
Metal
Ytterbium
Yb
173
70
70
103
70
2
Metal
Actinium
Ac
227
89
89
138
89
5
Metal
Molybdenum
Mo
96
42
42
54
42
6
Metal
Thallium
Tl
204
81
81
123
81
5
Metal
Fermium
Fm
257
100
100
157
100
8
Metal
Nobelium
No
259
102
102
157
102
2
Metal
Hydrogen
H
1
1
1
0
1
1
Non-metal
Carbon
C
12
6
6
6
6
4
Non-metal
Nitrogen
N
14
7
7
7
7
6
Non-metal
Barium
Ba
137
56
56
81
56
4
Metal
Helium
He
4
2
2
2
2
2
Non-metal
Calcium
Ca
40
20
20
20
20
2
Metal
Silicon
Si
28
14
14
14
14
4
Metalloid
Argon
Ar
40
18
18
22
18
8
Non-metal
Magnesium
Mg
25
12
12
13
12
2
Metal
Seaborgium
Sg
265
106
106
159
106
6
Metal
Periodic Table Puzzle - Answer Key
Down:
1. IRON - I have 26 protons.
3. HYDROGEN - I am not really an alkali metal, but since I have only 1 electron I behave like
them.
4. NICKEL - I am a metal with 28 electrons.
7. ALUMINUM - I am a member of the boron family and am the most abundant metal in the
Earth’s crust.
8. OXYGEN - I am a gas with 8 protons and 8 neutrons.
10. LEAD - I am a member of the carbon family often mistaken for the end of your pencil.
12. MERCURY - I am a metal that is liquid at room temperature.
14. SILVER - My atomic number is 47 and I am used to make photographic film.
15. CALCIUM - I have 20 neutrons and am found in your teeth and bones.
16. PHOSPHORUS - I am a member of the nitrogen family with 16 neutrons.
18. FLUORINE - I am a gas with a mass number of 19.
19. POTASSIUM - I am the first element in the fourth period used in making fertilizer.
22. TIN - You can find me in the carbon family in the fifth period.
Across:
2. CHLORINE - My atomic mass is 35.453.
5. SULFUR - I have 2 electrons in the first shell, 8 in the second shell, and 6 in the third shell.
6. CARBON - I am the head of the carbon family known as the “basis of life”.
9. GOLD - My atomic number is 79.
11. MANGANESE - I am a transition metal with 25 electrons.
13. NITROGEN - I make up 78% of the air and am found in the 15th group.
14. SODIUM - I am a silvery white metal used to make salt.
17. MAGNESIUM - I am a member of the alkaline earth metals used to make fireworks and
medicines.
20. HELIUM - I am a noble gas with 2 electrons.
21. SILICON - I am the 2nd most abundant element in the Earth’s crust and have 14 neutrons.
23. IODINE - I am a member of the halide family with an atomic number of 53.
24. ZINC - I am a transition metal with 30 electrons useful in making paint.
25. BROMINE - I am the only element in the halide family that is a liquid.
History of the Atom Notes
Fill in the following table:
Who and When
What
Democritus
400 BC
Said that all matter was made out of atoms. He
didn’t have any evidence to back up his model.
Aristotle
350 BC
Said that all matter was a combination of the
Four Essential Elements: Air, Fire, Water and
Earth. He didn’t have any evidence to back up
his model, but his idea was accepted as truth
for 2,000 years.
Said that all matter was composed of atoms.
AN atom was a solid, positively charged particle
(like a jaw breaker or a marble). Dalton is
credited with discovering the proton. Dalton
had evidence to back up his model.
Using cathode rays, he discovered the electron
- a negatively –charged particle in the atom.
Thomson’s model of the atom was of a chocolate
chip cookie. The cookie part was the positively
charged stuff from Dalton’s Model and the
chocolate chips were the electrons embedded in
the cookie.
Performed the famous “Gold Foil Experiment”.
He discovered the nucleus. Rutherford’s model
of the atom was of a nucleus (where the protons
were located) in the center with electrons
orbiting around it.
Discovered the neutron. The neutrons were
determined to be in the nucleus of the atom.
John Dalton
J.J. Thomson
Ernest Rutherford
Chadwick
Niels Bohr
Developed the Bohr Model of the Atom where
you had a nucleus with protons and neutrons and
the electrons orbiting around it in specific
orbitals. Electrons couldn’t be just anywhere.
Werner Heisenberg
Their discoveries lead to the modern “Electron
and Erwin Schrödinger Cloud Model of the Atom”. In this model the
electrons are everywhere, but the most likely
place to find them is in Bohr’s orbitals.
Name:
Hour:
Date:
Chemistry: Atomic Number and Mass Number
Complete the following chart and answer the questions below.
Number of
Protons
Atomic
Number
Element
Name
b
r;
8
ff
8
fb
I
{
0
1
G
6
hydrogen
Grbot.,
hydrogen
(
nitrogen
(
'1
(
{
"'rclh:>~~
U rq t\ ,'t.d",.,
~0
cesium
~
!~
L.\""-
11
tungsten
13 ~1V\.,'ho
&V'~.'4 """"'
silver
3
2
'7
14
2
146
d-3R'
S'~
82
( :Sf)
LJ
12
J.3
9~
L('J
47
b I
108
'It{
SS-
'1t.{
110
toL(
.ss-
45
80
24
~r
52
(,3
63
89
152
L(')
l1"J
bO
107
0(,
76
CJ"S "","1'tA"-.
14
1
ex V(
c..kto~'~~
12
0
I
92
~; lvev-
Mass Number
b
carbon
O'A.,.. .
. Numberof
Neutrons
ICfo
114
How are the atomic number and the number of protons related to each other?
Ato~~/c. 41
r
=: -4 of-
Al:>
fot\.~
How do the number of protons, number of neutrons, and the mass number relate to each other?
p rcf"ot'\.5
-+
p\eUft'"D1/\.5
=-
f\-.Pcs s 'f:/
What is the one thmg that determines the identity of an.atom (that is, whether it is an oxygen atom or a carbon
atom, etc.)?
n<>-
p.--"" 10",,-
Atomic Theory and the Periodic Table
L
M
F
S
o
M6NO
o
L
o
B
7
H
R...
0
H
o
10
:r
12
11
EN6Reo)'
r:
0
b
L
l--l
T
0
s
o
6
E
tV
.s
T
0
(]
oN
.
•
'
The Great Periodic Table/Atomic M&M Treasure Huntl
Today you are going on a treasure
hunt. Your goal is to unlock the secrets of the Periodic Table!
Each clue, each new discovery will unlock the Periodic Table's secrets and lead to more clues
which will lead you to even deeper mysteries
and even greater
when I say that the Periodic Table is the single most important
The Periodic Table is the ingredients
understand
understandings!
scientific
I am not kidding
document ever made.
list for the Universe - if you understand
how it works, you
the basis of EVERYTHING! Good luck and, as the great Sherlock Holmes would say,
"The hunt is on!"
Part One: The Plan
The plan for you today is to build Bohr Models of atoms using your Periodic Table and M&Ms.
Think of today as your chance to put everything
take your understanding
together
that we have learned so far and to
of the Periodic Table to new levels.
Materials
Each person will need to get 60 M&Ms for this lab (3 different
colors, 20 of each color).
Each person will need to put his or her M&Ms a double layer of paper towels.
One large sheet of white paper per person (on the workbench in the lab area).
Each person will need his/her
Periodic Table.
DO NOT EAT ANY M&Ms UNTIL YOUR ENTIRE GROUP IS DONE WITH THE LABII!!
Part Two: The Set-Up
1. Get all of your materials.
2. Divide your M&Ms into three groups (based on color). Label one group protons, one group
neutrons and the last grou'p electrons.
3. Draw three big concentric circles on your white sheet of paper. The smallest should be at
least 4" across. These will represent
the energy levels where the electrons
go.
Part Three: The Lab
1. You are going to fill out the information
build the model first
on the tables on the next 2 pages. You need to
and then fill out the corresponding
Hydrogen as an example for you.
2. While you are going through this activity,
part of the table. I have done
look for any connections between the element
model and its' placement on the Periodic Table.
3. Use your Periodic Table as necessary.
4. Work with your partners. Don't get ahead of your lab group!
NOTE: When your 9f.Q.MP has filled out the tables,
cleared to eat your M&Msl
you are
Element
Cl
7J
c
:E
~
0
3
~
~
H
Li
Be
B
C
N
Bohr Model
Drawing
1 1
1
2
lP~
On
Atomic
#
Ie
~~~
I~
J
2
3
2
4p \
~
S,.,
)
2
2
"
6
F
7
2
Atomic
Mass
#of
Neutrons
# of Energy
Levels
# of Valence
Electrons
1
1
1
1
0
1
1
3
3
3
7
4
2
1
4
4
4
9
5
2
2
5
5
5
11
6
2
3
6
6
6
12
6
2
4
7
7
7
14
7
2
5
8
8
8
16
8
2
6
9
9
9
19
10
2
7
10
10
10
20
10
2
8
)
'),
t 5~)L
'I
#of
Electrons
'\
r-,p ')
5
#of
Protons
)
J...~ 3e.
b
4
t
\
J
~p
2
b""l
a
Ne
0
)
~
~
/
/
"1f ) ')
~t., J..~ ~e
J J
qpJ
8
2
2
\
;)<!
(~)
top'
"
:<t2.
(o~
)
1~
)
\
z~
/
Build models of the elements below. Fill out the table as you go along. Work with your group - don't get ahead of your
partners! Look for any patterns/similarities
with an element and its' place on the Periodic Table.
Element
]:J
0
~~
c
:E
3
=l:t:
~
Na
Mg
AI
Si
Bohr Model
Drawing
Up '\
1
3
t~
Atomic
#
'\
~Q..
#of
Proton
s
#of
Electrons
Atomic
Mass
#of
Neutrons
# of Energy
Levels
# of Valence
Electrons
'\
(e.-
II
11
11
23
12
3
1
12
12
12
24
12
3
2
13
13
13
27
14
3
3
t{f<-
14
14
14
28
14
3
4
5~
15
15
15
31
16
3
5
16
16
16
32
16
3
6
17
17
17
35
18
3
7
18
18
40
22
3
8
~
VI) ) )
l~p'1 "\ \
2
3
fd
'" ) ))
l~p
3
3
,
3
,
~~
\
1«., ~~ ,~ ~"')
4
1e
~C2.
~
r"p ~) )&-
"\
J
('1Y1
P
5
3
5
6
3
CI
7
3
Ar
8
3
{Sf'
{6Y1
\ \~ ~
~~
)ft-
(b? '\
(6
')
2..e. ~e... ~ e..
h )
-) )
{~f
(<l
')
\
'\
~
;<e. ~ e 011:~ J /
)
lif' '" \
\,
~~7 ~~.
~)~
18
,
Part Four: Searching For the Clues
In this section, you are going to look for patterns/similarities/trends
in the rows and the
columns of the Periodic Table. Use your tables from above and your Periodic Table to answer
the following questions. Look for clues in the numbers and in your drawings.
Part 1: The Rows
The two tables that you filled out are for the 2nd and 3rd rows of the Periodic Table. Look at
each row carefully.
1. What thing(s) remain the same for each element in a given row?
The number of energy levels remains the same for elements in the same row.
2. A trend or pattern
the temperature
is a regular, consistent
change (i.e. as we go from winter to summer,
trends upward). What trends or patterns
do you see as you go from left
to right across the 2nd row? (Find at least three.)
The number of protons, electrons
and valence electrons
all increase by one as you go
across the row. The atomic mass increases and the number of neutrons generally
increases.
3. Do the trends and patterns
that you discovered
in question #2 hold true for the third
row of the Periodic Table, too?
Yes
4. Look over the rest of the Periodic Table. Do your trends/patterns
hold true for all
elements?
Yes
5. Share your findings with the group next to you. Did they find the same clues? Write
down any new clues that they found.
6. Show your findings to the teacher and get his initials here:
_
Part 2: The Columns
The two tables that you filled out represent
columns 1-8 of the Periodic Table (only the first
few elements of each column).
1. What thing(s) remain the same for each element in a given column?
The all have the same number of valence electrons.
2. A trend or pattern
the temperature
is a regular, consistent
change (i.e. as we go from winter to summer,
trends upward). What trends or patterns
do you see as you go down a
column? (Find at least three.)
The number of energy levels goes up by one as you go down a column. The number of
protons, electrons
and neutrons increases as you go down a column.
3. Do the trends and patterns
that you discovered
in question #2 hold true for all of the
columns?
Yes
4. Look over the rest of the Periodic Table. Do your trends/patterns
hold true for all
elements in each column?
Yes
5. Share your findings with the group next to you. Did they find the same clues? Write
down any new clues that they found.
6. Show your findings to the teacher and get his initials here:
_
Part Five: Sharing Your Findings with the Class
For this part, we are going to come up with a class list of the trends, similarities
and
patterns that we discovered on the Periodic Table. Write down the agreed upon items below:
Row Clues:
Column Clues:
Part Six: Blazing a New Traill
Trends, patterns and similarities are no good if you can't use them. You are going to take what you have learned and predict
the properties of elements that haven't even been discovered yet! This is exactly what scientists do - they take what they
know and use it to help them discover new things! In the table below, I want you to tell me about the as yet undiscovered
elements from row 8 of the Periodic Table. To make things a little easier, we will only do the elements for columns 1 through
8 (the ones you numbered at the top of your Periodic table).
<1
0
1:J
0
c
:E
::s
:t:t:
3
:t:t:
1 8
2 8
3 8
4 8
5 8
6 8
7 8
8 8
.
Bohr Model Drawing
(draw the rings, but
only label the outside
ring electron number)
Atomic #
'\
119
))1)
120
p
133 )))))
~I)~ 133
13'fp jJ))) ~ ~~ 134
(3~p ))))))/
~
135
(~bF)))))
) ~~ 136
131t ))j)))) }- 137
(~rf I J))) )) ~e 138
,
f1~p ))))))
J~
)
f~Of
~I
~
#of
Protons
#of
Electrons
119 119
120 120
133 133
134 134
135 135
136 136
137 137
138 138
Atomic
Mass
#of
Neutrons
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
#of
Energy
Levels
8
8
8
8
8
8
8
8
#of
Valence
Electrons
1
2
3
4
5
6
7
8
Lewis - Dot Structures
In chemistry, the valence electrons are the most important thing to keep track of for an atom. A Lewis-Dot
structure is a way of showing just the valence electrons of an atom.
•
H
oe
He
•
0
Li
•
No
•
K
Things
-
•
• Be
• B•
•
•
• Mg
• AI
•
•
Q.,
• C.
• N.
'»
•
•
• Si •
. p.
•
••
•
-.
: o.
•
: F.
-.
: S.
••
•
••
-.
: CI••
••
~-
: Ne •·
••
:Ar:
••
•••
• Co
to know for this unit's test:
Knowthe history of the atom (who discovered what part)
Knowthe three parts of the atom, their charges, masses and where you find them)
Knowhow to draw Bohr Model Diagrams
Knowhow to draw Lewis-Dot Structures
Knowhow to read your Periodic Table (protons, neutrons, electrons, energy levels, valence electrons,
trends in rows and columns)
The Periodic Table of the Elements (with Ionization Energies)
1
18
Hydrogen
Alkali metals
Alkaline earth metals
Transition metals
Lanthanides
Actinides
Other metals
Metalloids (semi-metal)
Nonmetals
Halogens
Noble gases
1
H
1.01
1312
2
Lithium
Beryllium
3
4
Li
Be
6.94
9.01
520
Sodium
900
Element name
Helium
Mercury
2
Atomic #
80
He
Hg
Symbol
First ionization
energy (kJ/mol)
200.59
Avg. Mass
1007
13
14
15
16
17
Boron
Carbon
Nitrogen
Oxygen
Fluorine
2372
Neon
5
6
7
8
9
10
B
C
N
O
F
Ne
10.81
12.01
14.01
16.00
19.00
20.18
801
Magnesium
4.00
Aluminum
1087
1402
1314
Silicon
Phosphorus
Sulfur
1681
Chlorine
2081
Argon
11
12
13
14
15
16
17
18
Na
Mg
Al
Si
P
S
Cl
Ar
22.99
24.31
26.98
28.09
30.97
32.07
35.45
39.95
496
738
3
4
5
6
7
8
9
10
11
12
578
787
1012
1000
1251
1521
Potassium
Calcium
Scandium
Titanium
Vanadium
Chromium
Manganese
Iron
Cobalt
Nickel
Copper
Zinc
Gallium
Germanium
Arsenic
Selenium
Bromine
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
39.10
40.08
44.96
47.88
50.94
52.00
54.94
55.85
58.93
58.69
63.55
65.39
69.72
72.61
74.92
78.96
79.90
83.80
419
Rubidium
590
633
Strontium
Yttrium
659
Zirconium
651
Niobium
653
717
763
Molybdenum
Technetium
Ruthenium
760
Rhodium
737
Palladium
746
Silver
906
Cadmium
579
Indium
762
Tin
947
Antimony
941
1140
Tellurium
Iodine
Krypton
1351
Xenon
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
85.47
87.62
88.91
91.22
92.91
95.94
(98)
101.07
102.91
106.42
107.87
112.41
114.82
118.71
121.76
127.60
126.90
131.29
710
720
868
558
709
403
Caesium
550
Barium
55
56
Cs
Ba
132.91
137.33
376
503
Francium
Lutetium
57-70
*
Radium
87
88
Fr
Ra
(223)
(226)
380
600
**
509
*lanthanides
Tantalum
684
Tungsten
702
Rhenium
Osmium
Iridium
804
Platinum
731
Gold
Mercury
Thallium
Lead
834
Bismuth
869
Polonium
1008
Astatine
1170
Radon
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
174.97
178.49
180.95
183.84
186.21
190.23
192.22
195.08
196.97
200.59
204.38
207.20
208.98
(209)
(210)
524
659
770
760
840
880
870
890.1
1007
589
716
703
812
890
1037
Hassium
Meitnerium
Darmstadtium
Roentgenium
Copernicium
Ununquadium
Ununpentium
Ununhexium
Ununseptium
Ununoctium
Rutherfordium
761
Dubnium
Seaborgium
Bohrium
Ununtrium
(222)
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
Lr
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Cn
Uut
Uuq
Uup
Uuh
Uus
Uuo
(262)
(267)
(268)
(271)
(272)
(270)
(276)
(281)
(285)
(284)
(289)
(288)
(293)
(294?)
(294)
---
---
---
Ytterbium
470
580
---
---
---
---
---
(280)
---
---
---
---
---
Lanthanum
Cerium
Praseodymium
Neodymium
Promethium
Samarium
Europium
Gadolinium
Terbium
Dysprosium
Holmium
Erbium
Thulium
57
58
59
60
61
62
63
64
65
66
67
68
69
70
La
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
138.91
140.12
140.91
144.24
(145)
150.36
151.97
157.25
158.93
162.50
164.93
167.26
168.93
173.04
538
534
545
547
573
581
589
Actinium
**actinides
Hafnium
652
Lu
Lawrencium
89-102
640
Thorium
527
533
Protactinium
Uranium
540
Neptunium
Plutonium
Americium
593
Curium
566
Berkelium
Californium
Einsteinium
Fermium
597
Mendelevium
603
Nobelium
89
90
91
92
93
94
95
96
97
98
99
100
101
102
Ac
Th
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
(227)
232.04
231.04
238.03
(237)
(244)
(243)
(247)
(247)
(251)
(252)
(257)
(258)
(259)
499
587
568
598
605
585
578
581
601
608
609
627
635
642
---
The Periodic Table of the Elements (including Atomic Radius)
1
18
Hydrogen
Alkali metals
Alkaline earth metals
Transition metals
Lanthanides
Actinides
Other metals
Metalloids (semi-metal)
Nonmetals
Halogens
Noble gases
1
H
1.01
31
2
Lithium
Beryllium
3
4
Li
Be
6.94
9.01
128
96
Element name
Helium
Mercury
2
Atomic #
80
He
Hg
Symbol
200.59
Atomic radius
(picometers)
Avg. Mass
14
15
16
17
Boron
Carbon
Nitrogen
Oxygen
Fluorine
28
Neon
5
6
7
8
9
10
B
C
N
O
F
Ne
10.81
132
4.00
13
12.01
84
14.01
76
16.00
71
19.00
66
20.18
57
58
Sodium
Magnesium
Aluminum
Silicon
Phosphorus
Sulfur
Chlorine
11
12
13
14
15
16
17
18
Na
Mg
Al
Si
P
S
Cl
Ar
22.99
24.31
26.98
28.09
30.97
32.07
35.45
39.95
166
141
3
4
5
6
7
8
9
10
11
12
121
111
107
105
102
Argon
106
Potassium
Calcium
Scandium
Titanium
Vanadium
Chromium
Manganese
Iron
Cobalt
Nickel
Copper
Zinc
Gallium
Germanium
Arsenic
Selenium
Bromine
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
36
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
39.10
40.08
44.96
47.88
50.94
52.00
54.94
55.85
58.93
58.69
63.55
65.39
69.72
72.61
74.92
78.96
79.90
83.80
203
Rubidium
176
170
Strontium
Yttrium
160
Zirconium
153
Niobium
139
139
132
Molybdenum
Technetium
Ruthenium
126
Rhodium
124
Palladium
132
Silver
122
Cadmium
122
Indium
120
Tin
119
Antimony
120
120
Tellurium
Iodine
Krypton
116
Xenon
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
Rb
Sr
Y
Zr
Nb
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
I
Xe
85.47
87.62
88.91
91.22
92.91
95.94
(98)
101.07
102.91
106.42
107.87
112.41
114.82
118.71
121.76
127.60
126.90
146
142
144
142
139
220
Cesium
195
Barium
55
56
Cs
Ba
132.91
137.33
244
215
Francium
Radium
87
88
Fr
Ra
(223)
(226)
260
190
Lutetium
57-70
*
**
Tungsten
147
Rhenium
Osmium
Iridium
139
Platinum
145
Gold
Mercury
Thallium
Lead
139
Bismuth
138
Polonium
139
Astatine
131.29
140
Radon
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
Hf
Ta
W
Re
Os
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
174.97
178.49
180.95
183.84
186.21
190.23
192.22
195.08
196.97
200.59
204.38
207.20
208.98
(209)
(210)
187
175
162
151
144
141
136
136
132
145
146
148
140
150
150
Ununoctium
170
(222)
Rutherfordium
Dubnium
Seaborgium
Bohrium
Hassium
Meitnerium
Darmstadtium
Roentgenium
Copernicium
Ununtrium
Ununquadium
Ununpentium
Ununhexium
Ununseptium
103
104
105
106
107
108
109
110
111
112
113
114
115
116
117
118
Lr
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Cn
Uut
Uuq
Uup
Uuh
Uus
Uuo
(271)
(272)
(270)
(276)
(281)
(285)
(284)
(289)
(288)
(293)
(294?)
(294)
---
---
---
Ytterbium
(262)
(267)
---
(268)
---
---
---
---
---
---
(280)
---
---
---
---
---
Lanthanum
Cerium
Praseodymium
Neodymium
Promethium
Samarium
Europium
Gadolinium
Terbium
Dysprosium
Holmium
Erbium
Thulium
57
58
59
60
61
62
63
64
65
66
67
68
69
70
La
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
138.91
140.12
140.91
144.24
(145)
150.36
151.97
157.25
158.93
162.50
164.93
167.26
168.93
173.04
207
204
198
198
192
192
189
Actinium
**actinides
Tantalum
154
71
221
*lanthanides
Hafnium
164
Lu
Lawrencium
89-102
175
Thorium
203
201
Protactinium
Uranium
199
Neptunium
Plutonium
Americium
196
Curium
194
Berkelium
Californium
Einsteinium
Fermium
190
Mendelevium
187
Nobelium
89
90
91
92
93
94
95
96
97
98
99
100
101
102
Ac
Th
Pa
U
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
(227)
232.04
231.04
238.03
(237)
(244)
(243)
(247)
(247)
(252)
(257)
(258)
215
206
200
196
190
187
180
169
---
(251)
---
---
---
---
(259)
---
---
Name______________________
Graphing the Periodic Table
Define the following:
GROUPS:
Groups are also called columns or families.
PERIODS:
Periods are also called rows.
Background/Explanation:
The atomic radius is the distance from the nucleus of an atom out to the furthest
valence electron. The atomic radius tells you the size of an atom. The larger the
radius, the larger the atom.
Procedure:
1. Use the Periodic Table provided to graph the Atomic Radius vs. the
Atomic Number for elements 3-20.
2. Use the Periodic Table provided to graph the Atomic Radius vs. the
Atomic Number for Groups 1 and 2.
Graph 1:
Atomic Number Vs Atomic Radius for elements 3-20
Questions for Graph #1:
1. Within a period, as the atomic number increases, the radius of an atom
__decreases___.
2. What causes your graph to “jump” from elements 10 to 11 and 18 to 19? (Hint:
Look at your Periodic Table!)
You are starting a new row on the Periodic Table.
3. What is periodic (look for repeating patterns) about your graph?
At the beginning of a row, the atomic radius is the highest and it goes down as you
go across the row.
Graph #2: Atomic Number vs. Atomic Radius for Groups 1 & 2
Questions for Graph #2:
4. Within a group, as the atomic number increases, the atomic radius
__increases_____.
5. Which generally has larger atoms Group 1 or Group 2? __Group 1____
6. Where do you think Group 3 would belong on this graph? Draw a dashed line in
this location.
Group 3 would be a curve just like groups 1 & 2, except that it would be beneath
them.
Analysis Questions (questions for BOTH graphs):
7. Going down a group, what is the trend for atomic size?
The atom radius increases.
8. Explain the trend form question #7. Give reasons for why it happens.
Every time you go down one element in a column, you are adding another
energy level to the atom.
9. Going across a period, what is the trend for atomic radius?
When you go across a period the atomic radius decreases.
10. Explain the trend form question #9. Give reasons for why it happens.
It doesn’t make sense that the atomic radius decreases as you go across a row,
because at the same time you are adding more stuff (protons, neutrons and
electrons) to each atom. You have to remember that the protons and electrons are
oppositely charged and that means that they are attracted to each other. You
also have to remember that atoms with full outside energy levels (or 8 electrons
in the outside energy level are more stable than other atoms. A more stable atom
means that the atom is in a lower energy state and that means that the electrons
are actually closer to the nucleus (where the protons are located).
A Bonus Trend:
Look at the graph below and answer the questions that follow.
Boiling & Melting Points of a Group
1. What happens to the boiling and melting points as you go down this column?
As you go down the column, the boiling points and melting points increase.
2. Which elements are gases at room temperature?
Fluorine and chlorine are gases at room temperature.
3. Which element would be a liquid at room temperature?
Bromine is a liquid at room temperature.
4. Which element is a solid at room temperature?
Iodine is a solid at room temperature.
5. On the planet Vulcan, room temperature is -20 °C. What state will bromine be in
at that temperature? Bromine will be a solid at that temperature.
Ions and Ionization Energy
Today you are going to learn a little bit more about atoms and, in turn, the
Periodic Table. You know that chemical reactions happen. You have seen fireworks
exploding, wood burning, food baking and much more. The driving question here is
“Why do chemical reactions happen?”
To answer this question we will have to cover a few points:
Point #1:
Atoms react, and reactions happen, because atoms want to be “happy”.
Here is what makes an atom happy:
- Atoms are happy when they have a full outside energy level of electrons
or 8 valence electrons.
- Some atoms can be happy when they have ZERO electrons.
The reason that this makes atoms “happy” is that when atoms have the outside
energy level full or have 8 valence electrons they are the most stable. This is
their lowest energy state.
Point #2:
Atoms gain or lose electrons in order to reach a state of “happiness”.
Every atom wants to be just like the Noble Gases. To do this they need to gain or
lose electrons. Atoms straight off of the Periodic Table have the same number of
protons (+ charges) and electrons (- charges). The charges cancel out and the
result is that all atoms on the Periodic Table are neutral or have no charge.
Here is an example for you:
Lithium has an atomic number of 3. This means that it has 3 protons (for an overall charge of “+3”) and 3 electrons (for an over-all charge of “-3”). When you add
the charges together you get a net charge of zero (“+3” + “-3” = 0).
As you can see, a lithium atom straight off the Periodic Table has no charge (it is
neutral), but it is it “happy”? Well, according to the rules, it has to have a full
outer energy level or 8 electrons in the outer energy level. Let’s look at the Bohr
Model for Lithium:
If you look at the model, you will notice two things:
1. Lithium has one electron in the outer energy level – it isn’t “happy”.
2. Lithium has a full inner energy level.
If lithium wants to be happy, it has two options it can gain 7 electrons in order to
fill up the outside energy level or it can lose the one electron that it has in the
outer level and then it will be left with just its inner energy level which is full.
What will lithium do? Will it lose 1 electron or gain 7 more electrons? To answer
that question we need to get to Point #3.
Point #3:
Atoms are ‘lazy”.
Atoms will do the least amount of work possible (this reminds me of some
students that I know). Lithium is faced with the decision of losing one electron or
gaining 7 new ones. Being lazy, it will go with losing one electron. When it does
that, you get a Bohr Model that looks like this:
Lithium has two electrons in the outside energy level and, since it is the first
energy level which only can hold 2 electrons, lithium is, by definition, happy.
When you look at the model above, you will note that the happy lithium has a “+”
sign by it. That is because the lithium atom is charged now – it has become an ion
and that leads us to Point #4.
Point #4:
Ions are charged atoms.
Let’s look at lithium before it was happy (right off the Periodic Table) and after it
became happy (when it lost the electron).
Before:
Lithium has 3 protons (+3 charge) and 3 electrons (-3 charge). The over-all charge
of the atom is zero (“+3” + “-3” = 0).
After:
Lithium still has 3 protons (+3 charge), but now it only has 2 electrons (-2 charge).
The over-all charge is +1 (“+3” + “-2” = +1).
Practice Problems
1. For each of the elements listed on the table below, write down how many
valence electrons it has, what it could do to become happy, what it will do to
become happy and what charge ion it will form.
Element
Valence
What it could do
Electrons to become happy
Lithium
1. Gain 7 electrons
1
2. Lose 1 electron
Beryllium
1. Gain 6
2
2. Lose 2
Boron
1. Gain 5
3
2. Lose 3
Carbon
1. Gain 4
4
2. Lose 4
Nitrogen
1. Gain 3
5
2. Lose 5
Oxygen
1. Gain 2
6
2. Lose 6
Fluorine
1. Gain 1
7
2. Lose 7
Neon
1. Gain 0
8
2. Lose 0
Hydrogen
1. Gain 1
1
2. Lose 1
Francium
1. Gain 7
1
2. Lose 1
Iodine
1. Gain 1
7
2. Lose 7
What it will do to Ion Charge
become happy
(Show work!)
Lose 1 electron
#P + #E
(+3) + (-2) = +1
Lose 2 electrons
(+4) + (-2) = +2
Lose 3 electrons
(+5) + (-2) = +3
Gain 4 electrons
Lose 4 electrons
(+6) +(-10) = -4
(+6) + (-2) = +4
(+7) + (-10) =
-3
(+8) + (-10) =
-2
(+9) + (-10) =
-1
(+10) + (-10) =
0
(+1) + (-2) = -1
(+1) + (-0) = +1
(+87) + (-86) =
+1
(+53) + (-54) =
-1
Gain 3 electrons
Gain 2 electrons
Gain 1 electron
Nothing
Gain 1 electron
Lose 1 electron
Lose 1 electron
Gain 1 electron
2. On the table below, fill out how each type of atom typically becomes an ion:
Type of Element
Metals
Non-metals
Metalloids
Become ions by ……..
Losing electrons
Gaining electrons
Gaining or losing electrons
3. What is so special about the elements in the Carbon Family?
They can either gain or lose electrons.
4. Hydrogen can do two things to become “happy”. Based upon that, what other
column on the Periodic Table could Hydrogen be placed in?
Column #7 (since it can form an ion with a charge of “-1”).
Alright, so now you know that atoms want to be “happy” and what atoms will do to
become “happy”. Some atoms gain electrons and others lose them. Some atoms will
do either. This leads us to a new property of atoms to learn about and a new trend
to discover on your Periodic Table:
First Ionization Energy
First ionization energy is defined as the amount of energy that it would take to
remove one valence electron from an atom. Based upon what you have learned
about atoms and ions so far, it is time to make a prediction.
Prediction (circle your choice in the sentence below)
Atoms that gain/lose electrons will have the highest first ionization energies.
Why did you make the choice that you made?
Graphing First Ionization Energy
Use the Periodic Table provided below to make the following graphs.
Graph #1:
First Ionization Energy vs. Atomic Number for Elements 1-20
1. What is the trend for first ionization energy as you go across a period?
It increases.
2. Why do you think it does this?
Going across a row/period, you go from atoms that want to lose electrons (metals)
to atoms that want to add on more electrons. It makes sense that an atom that
wants to get rid of electrons wouldn’t hold on to them as tightly as an atom that
needs to gain electrons to become stable.
Graph #2
First Ionization Energy vs. Atomic Number for Groups 1&2
First Ionization Energy vs. Atomic
Number for Groups 1 & 2
1400
1200
Ionization Energy
1000
800
Column 1
600
Column 2
400
200
0
1
2
3
4
5
6
7
3. What is the trend for first ionization energy as you go down a group?
It decreases.
4. Why do you think it does this?
Each time you go down a column, you are adding an extra energy level. The
attraction between the nucleus and the electrons decreases as they move
away from each other.
5. How might first ionization energy help you explain why the atomic radius
(yesterday’s trend) of the atoms decreases as you go across a period?
6. Atoms that gain electrons in order to become “happy” (get a full outer
energy level or have 8 electrons in the outer level) tend to hold on to their
electrons tighter. They pull them closer to the nucleus and that make sit
harder to pull them away.






