Science Test Review Sheet: Chemistry and Matter

Science Test Review Sheet: Chemistry and Matter
*If you can answer these questions, then you can do very well on the test.
1) Give an example of a molecule.
2) Give an example of an atom.
3) Give an example of an element.
4) Give an example of a compound.
5) How are chemical formulas similar to a food recipe?
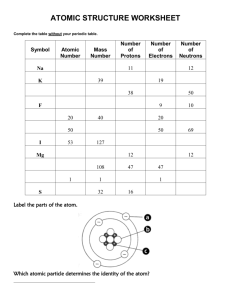
The next 3 questions relate to the energy level diagram pictured below:
6) What is the chemical name for the atom? (hint: count the electrons, then use your periodic table)
7) What is the atomic symbol for the atom? (use the periodic table)
8) How many electrons, protons, and neutrons does this atom have? (you need to use your periodic table)
9) What particles are in the nucleus of an atom?
10) What charge does each of the following have: electrons, protons, neutrons?
11) Which particles (protons, electrons, neutrons) are heavy (and have mass)?
12) If an element has 15 protons, now many electrons does it have?
13) If an element has an atomic mass of 55.85 and an atomic number 26, how many neutrons does it have?
14) What element has atomic number 26?
15) What is the chemical name for the element with atomic symbol Hg?
16) What is the definition of matter ?
17) What is the definition of mass ?
18) What is an ion ?
19) What is the difference between a chemical change and a physical change? Give some examples of each.
20) How many electrons does the K energy level hold? How many does L hold? How many does M hold?
21) Draw the energy level diagram for the element Phosphorus.
22) What did alchemists try to do?
23) How is a solution different from a mixture? Give an example of a mixture, and an example of a solution.
24) What does the word dissolve mean?
25) When sugar dissolves in water, which is the solute and which is the solvent ?
26) What is surface tension ?
27) Water molecules are polar . What does this mean?
28) When we mixed baking soda with vinegar, what chemical was produced? (hint: what caused the top to blow off of the film container)
29) What does the Law of Conservation of Matter state?
30) What is the definition of a chemical reaction ?
31) What can scientists add to a reaction to make chemicals react? (hint: there are two thing)
32) In our “Egg in Vinegar”” lab, what happened to the egg shell after it sat in vinegar for 3 days? What chemical in the eggshell did the vinegar react with?
There will 3 bonus marks for balancing a chemical equation on the test.
Try to balance the following equation:
______ AgI + ______ Na
2
S → ______ Ag
2