Continuous, Emission, and Absorption Line Spectra
advertisement

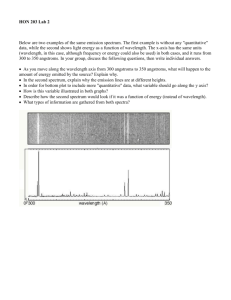
name Continuous, Emission, and Absorption Line Spectra In this lab we will be looking at emission and absorption lines and understanding what type of information we can gain from them. There are four parts to this lab. a) b) c) d) Building your own Spectroscope Looking at a Light Bulb and analyzing Black Body Radiation Looking the Solar Spectrum Looking at different types of Fluorescent Light Tubes Below is a graphical illustration of the differences between Continuous Spectra, Emission Line Spectra and Absorption Line Spectra (this is also known as “Kirchoff’s Laws”). Make sure you understand the concept of this. If you are still uncertain about what it means revisit your PreLab. Figure found widely on the internet, e.g. http://cfa-www.harvard.edu/~jbattat/a35/cont_abs_em.html Original figure from the book “Universe” by Freedman and Kaufmann This lab utilizes materials by Project STAR (http://www.starlab.com/psmain.html). Spectral Line Formation Lab 8 1 Part I — Building a Spectroscope Go to Site http://www.starlab.com/psprod.html and click on Cardboard Spectroscopy Kit. Or get the spectroscopy kit from your instructor. The texts below are the instructions from that kit. 2 Lab 8 Spectral Line Formation Spectral Line Formation Lab 8 3 Part II — Lamps and Black Bodies 1) Observe the Intensity and the Color of the Light from the light bulb. Using the Variable Switch turn on the Light Bulb and slowly increase the Voltage. Do not use the spectroscope. What happens to the intensity as you increase the electricity? What happens to the temperature as you increase the electricity? What happens to the color as you increase the electricity? 2) Use the Spectroscope to analyze the light from that bulb. Pick an intermediate Intensity and describe what you see. List the colors from left to right. Using color pens, draw what you see. Identify and label wavelengths, i.e., write the appropriate wavelengths below the drawing. Draw an arrow in the direction of increasing wavelengths. 4 Lab 8 Spectral Line Formation 3) Drawing the Spectrum in the form of a graph. Are all colors equally bright? Which color is brightest? _________________________________ Now turn the variable switch to a setting where yellow is the brightest color. Let’s draw a graph of what you see. In the previous drawing you looked at the colors, and labeled the wavelengths – this corresponds to the x-axis of your graph. The y-axis is the intensity of each color. This is shown below. very bright Intensity bright somewhat fainter much fainter Wavelength Compare the relative intensities of those colors. (How much fainter is the red light compared to the yellow? What about the blue light? Use the words “somewhat fainter” and “much fainter”.) Draw this into the above graph. (For example, for yellow draw a point the Intensity is “bright”; repeat for the other colors). Connect all pints with a smooth curve that goes through all those points. And voila – this is your spectrum – it the “Black Body Spectrum” (or the “Planck Spectrum” – same thing, other name). Now repeat the above analysis for two more settings – the case where the blue light is the most intense, and where orange is most intense. Draw those points into the above graph and connect the points of each of the two settings. In WORDS describe what happens to the shape of the black body spectrum as the electricity is turned down? Spectral Line Formation Lab 8 5 4) Understanding the Correlations In WORDS describe the correlation between the peak wavelength of the black body spectrum and the overall color of the light. In WORDS describe the correlation between the temperature and intensity of the light bulb. Write a formula for that correlation into the box below. This is “Stephan Bolzman’s Law”. If we were to make record the exact measurements of temperature and intensity, we would end up with a better mathematical description of that formula. Look up that version of Stephan Bolzman’s Law and write it into the box below. In WORDS describe the correlation between temperature and the peak wavelength of the black body spectrum (denote the peak wavelength by λmax). Write a formula for that correlation into the box below. This is “Wien’s Law”. If we were to make record the exact measurements of temperature and wavelengths, we would end up with a better mathematical description of that formula. Look up that version of Wien’s Law and write it into the box below. 6 Lab 8 Spectral Line Formation Part III — Gasses Go back to your lab and look at the spectra of the gas tubes. Draw which lines you see, and then identify the gases by comparing the spectra to the spectra in the appendix. Read off the wavelengths of the major emission lines and write those numbers in the space below your drawings. [Note you will only be able to see the numbers if you have used the black tape to block ambient light. But even then it will be tricky to read the scale since the gases are not very bright.] Gas: Hydrogen 7000Å 4000Å Å Wavelength 7000Å 4000Å Å Wavelength 7000Å 4000Å Å Wavelength 7000Å 4000Å Å Wavelength 7000Å 4000Å Å Wavelength 7000Å 4000Å Å Wavelength Gas 1: Gas 2: Gas 3: Gas 4: Gas 5: Spectral Line Formation Lab 8 7 1) When looking at the gasses, what types of spectra do you see? [continuous, emission line, absorption line spectra] Justify your answer. Explain how these spectra differ from the spectrum of a tungsten lamp in part II? 2) When it is dark, look at the street lamps all over the campus (you might have to continue with this part of the lab later, i.e., in the evening). Draw the spectrum of the lamps: Street Lamp 4000Å Then identify the gases in those lamps. Justify your explanation. 8 Lab 8 Spectral Line Formation 7000Å Wavelength Æ Do either Option 1 OR Option 2 [or both for extra credit] Part IV — Option 1 – The Sun and Black Bodies 1) Go outside and try out your spectroscope. Look at the Sun with your spectroscope. NEVER look directly at the Sun!!! 2) What type of spectrum do you see? [continuous, emission line, absorption line spectrum] Justify your answer. 3) Explain the nature and the origin of this light. a) First explain the cause of the “Black Body Radiation.” b) Then explain where (in which part of the Sun) the “Black Body Radiation” originates. c) Explain how the “Spectral Lines” in the sun are produced. d) Explain in which part of the Sun the “Spectral Lines” are formed. Spectral Line Formation Lab 8 9 Do either Option 1 OR Option 2 [or both for extra credit] Part IV — Option 2 – Do at night — The Moon 1) Look at the Moon with your spectroscope. 2) What type of spectrum do you see? [continuous, emission, absorption spectrum] Justify your answer. 3) Explain the nature and the origin of this light. a) What type of light do you see? Is it Black Body Radiation? Explain your answer. b) At what wavelength would the moon emit Black Body Radiation. Calculate this. c) To which part in the electromagnetic spectrum does this correspond? d) If you had a better spectroscope, do you think you might be able to see emission of absorption lines? Which ones? Explain your answer. 10 Lab 8 Spectral Line Formation Part V — Understanding Spectral Lines 1) Consider the Hydrogen gas. Draw the Bohr Model and indicate which line corresponds to which color. Write down the names of each line. (You should include the Lyman, Balmer and Paschen series and also indicate which lines are the Hα, Hβ, Hγ, and Hδ lines.) Which of these lines are seen in the visual part of the spectrum? What are their colors? Which of these lines is the strongest one? What is the overall color (without a prism or diffraction grating) of Hydrogen gas? 2) Why are the positions of the spectral lines different for different gases? (This is the reason why each gas has its own ‘fingerprint.’) Spectral Line Formation Lab 8 11 3) How can we use spectroscopy to determine the chemical make-up of Emission Line Nebulae? 4) Explain how you would determine the chemical composition of stars. 5) The atmospheres of different types of stars all have roughly the same chemical composition, but their spectra vary considerably. Explain WHY their spectra are different. 6) Explain how we use spectroscopy (not photometry) to determine the surface temperature of stars. 12 Lab 8 Spectral Line Formation Appendix: Emission Line Spectra of Elements Spectral Line Formation Lab 8 13 Obtained from http://www.amateurspectroscopy.com/color-spectra-of-chemical-elements.htm. 14 Lab 8 Spectral Line Formation