Composition of a Penny: Skill Builidng Lab
advertisement

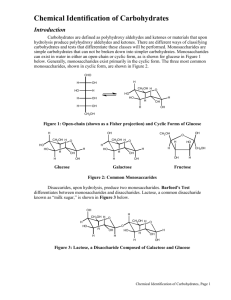
Chemical Reactions Carbohydrate Qualitative Analysis: Foundation Lab 2012, Sharmaine S. Cady East Stroudsburg University Skills to build: Doing microscale reactions Observing evidence of a chemical reaction Using chemical reactions to identify a carbohydrate Identifying reducing sugars Identifying aldoses and ketoses Identifying pentoses Introduction Along with proteins and fats, carbohydrates, or sugars, are one of the three main classes of food used to sustain life. They are essential components of all living organisms and constitute the most abundant class of biological molecules. The classification carbohydrate stems from the general molecular formula for monosaccharides, (C·H2O)n where n 3, which implies that these compounds are hydrates of carbon. However, carbohydrates are not true hydrates in the chemical sense. Carbohydrates, chemically, are polyhydroxy aldehydes (CHO) or ketones (C=O) or compounds which upon hydrolysis yield these compounds. Note that each carbon in a polyhydroxy aldehyde or ketone structure, except for the carbonyl functional group (in yellow), bears a hydroxyl (OH) functional group (in green). Polyhydroxy aldehydes and ketones with the same number of carbons are structural isomers of each other. H C O CH2OH H C OH C O H C OH H C OH H C OH H C OH CH2OH polyhydroxy aldehyde CH2OH polyhydroxy ketone Carbohydrate Qualitative Analysis Carbohydrates may be classified based upon the number of polyhydroxy aldehyde or ketone (saccharide) units found in their structure. The basic carbohydrate unit is a single polyhydroxy aldehyde or ketone, or monosaccharide. A disaccharide can be viewed as two monosaccharides from which water is removed to join the units. The bond that forms between the two sugar units is known as a glycosidic bond or linkage. Oligosaccharides consist of 3-10 sugar units covalently joined together, and polysaccharides are large biomolecular polymers of covalently bonded monosaccharide units. Monosaccharides Monosaccharides are further classified based upon the carbonyl functional group present and the total number of carbon atoms in the structure. The polyhydroxy aldehyde on the previous page contains five carbons and is classified as an aldopentose, where the prefixes aldo- and pent- indicate the aldehyde functional group and five carbon atoms, respectively. The polyhydroxy ketone structure is a ketopentose, where ketoindicates a ketone functional group. Monosaccharides may be represented by either a Fischer or Haworth projection. Fischer projections show the open chain form of the sugar, and Haworth projections show the cyclic form of the sugar. In a Fischer projection, a chiral carbon may be indicated by a pair of intersecting lines . In the Haworth projection, the chain of carbons forms a ring structure. The Fischer and Haworth projections for glucose are given below. The designation D indicates the stereoisomer where the hydroxyl group (green) at the highest numbered chiral carbon appears on the right in the Fischer projection. The symbol indicates the stereoisomer in which the hydroxyl group (blue) lies below the plane of the ring for the lowest numbered carbon in the ring (the anomeric carbon). When this hydroxyl group is shown above the plane, the designation is . Note that this hydroxyl group is formed from the carbonyl group during the ring closing reaction. Only the cyclic form is found in the solid state, while the linear form exists only at a low concentration in equilibrium with the and cyclic forms in solution. H 1 H HO H H 2 3 4 5 6 C O C OH C H C OH C OH CH2OH D-glucose 6 CH2OH H 5 H OH 4 OH 3 H O H H 1 2 OH OH -D-glucose 2 Carbohydrate Qualitative Analysis The cyclic structures of galactose, fructose, and ribose, are given below. CH2OH OH CH2OH O H H H OH H O H H D-galactose H H OH OH OH H O HO H OH CH2OH CH2OH OH OH H D-fructose H OH D-ribose Disaccharides Disaccharides are carbohydrates which upon hydrolysis (reaction with water) yield two monosaccharide structures. The most abundant disaccharide is sucrose, which is hydrolyzed to D-glucose and D-fructose. The two sugar units are covalently joined through an oxygen atom at carbons 1 and 2 on glucose and fructose, respectively. The covalent link between the two sugar units is referred to as an (12) CH2OH CH2OH H H OH H O H H OH OH OH H -H2O OH CH2OH OH O H H OH O glycosidic bond CH2OH O HO H CH2OH H OH O H H 1 H OH H HO 2 CH2OH H OH H sucrose glycosidic linkage. The structures of the disaccharides maltose and lactose are given below. Maltose contains two D-glucose units joined by an (1 4) glycosidic bond, while lactose consists of D-galactose and D-glucose joined by a (14) linkage. The glycosidic linkages are destroyed when disaccharides undergo hydrolysis back to the individual monosaccharides. 3 Carbohydrate Qualitative Analysis CH2OH H CH2OH CH2OH H H O H H 1 H OH CH2OH O H H H 4 OH O OH H OH H OH OH H OH O H 4 O H H H OH O OH 1 H H OH H OH H OH maltose lactose Polysaccharides Polysaccharides consist of large numbers of monosaccharides joined by glycosidic linkages. When the monosaccharide units are all identical, the molecule is referred to as a homopolysaccharide. If the monosaccharide units differ, the molecule is classified a heteropolysaccharide. Starch is a homopolysaccharide synthesized by plants for the storage of -D-glucose units and serves as a source of carbohydrates in animal diets. Plant starch occurs as a mixture of two different polymeric structural units. amylose is a linear polymer of several thousand glucose units joined by (14) glycosidic bonds. Amylopectin is a branched polymer with glucose connected in linear chains by (14) bonds and by (16) bonds at the branch points which occur on the average every 24 to 30 glucose units. Containing up to a million glucose residues, amylopectin is the larger of the two polymeric structures. Glycogen, the storage polysaccharide for glucose in animals and humans is structurally similar to amylopectin except that branching occurs every 8 to 12 glucose residues. CH2OH O H O CH2OH CH2OH H O H H O O H H 1 O 4H O H H O O H H O CH2OH H O H H O O H H O H O H H O H O amylose CH2OH O H O CH2OH H O H H O CH2OH O 1 O 4 H O H H O H O H H O 1 O O 6 O H H H 1 CH2OH O H O H H 4H O H H O CH2 H H O CH2OH O H O H H O O H H O H O H H O H O amylopectin 4 Carbohydrate Qualitative Analysis Carbohydrate Chemical Reactions The chemical reactions of carbohydrates is largely that of the hydroxy and carbonyl groups. The aldehyde and -hydroxy ketone (a ketone that has an OH on a carbon next to the carbonyl group) structural units in sugars undergo mild oxidation to carboxylic acids. In solution, the ring structure of sugars are able to open at the anomeric carbons to form the open-chain aldehyde or -hydroxy ketone form, which undergoes oxidation in the presence of a mild oxidizing agent. Such sugars are referred to as reducing sugars. However, when the anomeric ring carbon is involved in a glycosidic linkage, the ring is locked in place and cannot open for oxidation to occur. All monosaccharides are reducing sugars since no glycosidic linkages exist in their structures. The disaccharides maltose and lactose are reducing sugars since only one of the C1 anomeric carbons is involved in a glycosidic linkage, while sucrose is a nonreducing sugar since both the C1 and C2 anomeric carbons participate in the glycosidic linkage. Starch and glycogen are also nonreducing sugars because of their size. Molisch Test The Molisch test is used to determine the presence of carbohydrates, regardless of structure. The Molisch reagent provides the condensation reagent, -naphthol in alcohol. Concentrated sulfuric acid acts as the dehydrating reagent. It also serves as a catalyst for the hydrolysis of di- and polysaccharides. The presence of a purple ring at -naphthol OH O O CH2OH H O H H H OH OH OH ribose conc. H2SO4 H C O -naphthol O H -2 H2O O [O] C C OH OH furfural colorless dehydration product purple 5 Carbohydrate Qualitative Analysis the interface between the carbohydrate solution and concentrated sulfuric acid is a positive indication of a carbohydrate structure. Monosaccharides react more quickly than di- and polysaccharides, which must slowly undergo hydrolysis before they react with the Molisch reagent. Benedict's Test Benedict's reagent reacts with reducing sugars to form inorganic precipitates which are readily detected by visual observation. In the reaction between a reducing sugar and Benedict's reagent, copper(II) ion is reduced to copper(I) by the aldehyde functional group: H OH C O H C OH HO C H H C H C C O H C OH HO C H OH H C OH OH H C OH Cu2+ / OH(blue) CH2OH + Cu2O (red precipitate) CH2OH glucose gluconic acid The formation of a red to orange precipitate indicates the presence of a reducing sugar. Tollens's Test The Tollens's test also involves a mild oxidizing agent. In the Tollens's test, silver(I) ion is reduced to metallic silver. The formation of a silver mirror on the inside of the test tube indicates the presence of a reducing sugar. The Tollens's reagent must be freshly prepared to produce the proper results. H OH C O H C OH HO C H H C H C C O H C OH HO C H OH H C OH OH H C OH CH2OH glucose Ag(NH3)2+ + Ag (mirror) CH2OH gluconic acid Barfoed's Test Barfoed's reagent also uses the reduction of copper(II) ion to red Cu2O as an indication of a reducing sugar. However, it not as reactive as Benedict's reagent, and the 6 Carbohydrate Qualitative Analysis rate at which the red precipitate forms can be used to distinguish between monosaccharides and disaccharides. The appearance of red Cu2O within 2-3 minutes is a positive test for a monosaccharide; disaccharides produce the precipitate in approximately 10 minutes. Bial's Test The ability of the hydroxy groups to undergo dehydration reactions allows for identification of certain aldoses and ketoses. Concentrated acid is used to dehydrate the sugars, which produce colorless dehydration products. These dehydration products then react with a condensation reagent to form colored condensation products, which offer a visual means of positive identification. Figure 1 shows two condensation reagents commonly used to test for sugars. OH OH H3C OH OH orcinol resorcinol Figure 1. Condensation Reagents for Bial's and Seliwanoff's Tests Bial's test is used to distinguish a pentose from a hexose structure. The dehydrating agent is concentrated HCl, and the condensation reagent is orcinol in the presence of iron(III) chloride. Pentoses undergo dehydration to furfural, a cyclic aldehyde, which further reacts with orcinol to give a blue-colored condensation product. O CH2OH H O H H H OH OH OH ribose conc. HCl H C O orcinol blue condensation product -2 H2O furfural All other colored products are negative indicators for the presence of a pentose. Seliwanoff Test The Seliwanoff test differentiates between ketohexoses and aldohexoses. It also uses concentrated HCl for the dehydrating agent, but the condensation agent is resorcinol. Ketohexoses are dehydrated to 5-hydroxymethylfurfural, which undergoes condensation with resorcinol within two minutes to form a red-colored product. 7 Carbohydrate Qualitative Analysis H CH2OH O H H CH2OH O CH2OH conc. HCl OH -2 H2O HO OH H fructose C O resorcinol red condensation product 5-hydroxymethylfurfural The appearance of a peach color is not a positive test for a ketohexose. In this experiment, the above tests will be performed on glucose, ribose, fructose, galactose, sucrose, xylose, maltose, and lactose. The rates of reaction with the different reagents and the visual characteristics (color, intensity, amount of precipitate) of the products formed will be noted for comparison to an unknown carbohydrate sample. The flow chart may be used to identify the unknown carbohydrate as to class and structure. mono- or disaccharide Barfoed's test red ppt ~ 10 min red ppt. 2-3 min monosaccharide disaccharide Benedict's test no ppt. nonreducing disaccharide ( sucrose ) Bial's test other colors red ppt. pentose hexose reducing disaccharide ( lactose maltose ) blue Seliwanoff test red ketohexose ( fructose ) ( ribose xylose ) other aldohexose ( glucose, galactose ) Figure 2. Flow chart for unknown carbohydrate 8 Carbohydrate Qualitative Analysis Experimental Methods and Materials Safety considerations Wear suitable protective clothing, gloves, and eye/face protection! BENEDICT'S REAGENT eye, skin, and respiratory irritant harmful if swallowed; may cause gastrointestinal discomfort BARFOED'S REAGENT harmful if swallowed; may cause gastrointestinal discomfort corrosive to skin and eyes BIAL'S REAGENT corrosive to skin and eyes; harmful if ingested SELIWANOFF REAGENT corrosive to skin and eyes; harmful if ingested AMMONIUM HYDROXIDE contact may cause tissue damage to skin, eyes, mucous membrane, gastrointestinal & respiratory mucosa SILVER NITRATE damage to the eyes and can burn skin inhalation can irritate respiratory passages and mucous membranes ingestion can cause severe abdominal pain and gastroenteritis, that may be fatal SUGARS may cause eye irritation 9 Carbohydrate Qualitative Analysis Molisch Test Clean and thoroughly rinse with distilled water five test tubes. Label one for each of the five known carbohydrates. Place 10 drops of each sugar solution in its labeled test tube. Add 2 drops of the Molisch reagent to each test tube. Mix thoroughly with a glass stir rod. Be sure to clean the stir rod after each test tube. Hold the test tube at a 45 angle, and slowly and carefully add 20 drops of concentrated H2SO4 down the side of the test tube. The more dense acid will form a layer on the bottom of the test tube. Place in a rack and note the time for the appearance of a red to purple ring at the interface of the two layers. Aldopentoses, ketopentoses, and ketohexoses react more rapidly than aldohexoses and disaccharides. Record your results on your lab report. Benedict’s Test Clean and thoroughly rinse with distilled water five test tubes. Label one for each of the five known carbohydrates. Place 5 drops of each solution in its labeled test tube. Add 20 drops of Benedict's reagent to each solution. Mix thoroughly. Place all test tubes in boiling water at the same time. Note the time for the appearance of a red to reddish orange precipitate. After 1 minute remove the test tubes and place in a rack. Record your results. Barfoed's Test Prepare a boiling water bath(add boiling chips to prevent bumping). Clean and thoroughly rinse with distilled water five test tubes. Label one for each of the five known carbohydrates. Place 5 drops of each solution in its labeled test tube. Add 20 drops of the Barfoed's reagent to each test tube. Mix thoroughly. Place all test tubes in the boiling water at the same time. Note the time for the appearance of a red precipitate and remove the test tube. After 10 minutes remove all the test tubes and place in a rack. Record your results. Bial's Test Clean and thoroughly rinse with distilled water five test tubes. Label one for each of the five known carbohydrates. Place 5 drops of each solution in its labeled test tube. Add 20 drops of Bial's reagent to each test tube. Mix thoroughly. Place all test tubes in boiling water at the same time. Remove after 1 minute. A dark blue color indicates a pentose. Record your results. 10 Carbohydrate Qualitative Analysis Seliwanoff Test Clean and thoroughly rinse with distilled water five test tubes. Label one for each of the five known carbohydrates. Place 5 drops of each solution in its labeled test tube. Add 20 drops of Seliwanoff reagent to each solution. Mix thoroughly. Place all test tubes in boiling water at the same time. Note the time for the appearance of a cherry red color. Poly- and disaccharides hydrolyze slowly in the presence of acid and should take more time to give a positive test. After two minutes remove from the water and place in a rack. Record your results. Tollens's Test Clean and thoroughly rinse with distilled water three test tubes. Label one for glucose, one for sucrose, and one for lactose. Place 20 drops of 5% silver nitrate into a 100-mL beaker. Add 1 drop of 3 M NaOH to the beaker. Continue to add 2% ammonia with stirring to the beaker until the brownish precipitate of silver oxide just disappears. Divide the solution equally among the three labeled test tubes. Add 5 drops of each sugar solution to its labeled test tube. Do not mix. Place the test tubes in the boiling water bath for 2 minutes. Record your results. Identifying an Unknown Carbohydrate Obtain an unknown from your instructor and record its number on your lab report. Using the flow chart in Figure 2, determine the identity of your unknown. Do only those tests which are necessary to clearly establish the identity of the unknown. The possible unknowns are glucose, xylose, fructose, lactose, or sucrose. References Hendrickson, C. H.; Byrd, L. C.; Hunter, N. W. A Laboratory Manual for General, Organic, and Biochemistry, 3rd ed.; McGraw-Hill: Boston, 2001, pp 333-342. Pasto, D. J.; Johnson, C.R.; Miller, M. J. Experiments and Techniques in Organic Chemistry; Prentice Hall: Upper Saddle River, NJ, 1992, p 336. Pavia, D. L.; Lampman, G. M.; Kriz, G. S.; Engel, R. G. Introduction to Organic Laboratory Techniques: A Small Scale Approach, 2nd ed.; Brooks/Cole: Belmont, CA, 2005, pp 444-450. 11 Carbohydrate Qualitative Analysis Laboratory Report How accurate are the tests in identifying and classifying carbohydrates? Is your unknown a mono- or disaccharide? Is it a reducing or non-reducing sugar? 12