Grade 9 Science Exam Notes
advertisement

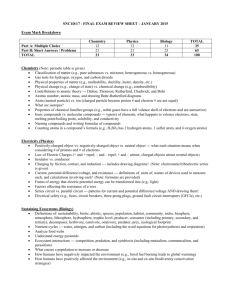
Grade 9 Science Exam Notes Syed Kamran Gordon Graydon Memorial Secondary School Syed Kamran Table of Contents Introducing Ecosystems ............................................................................................... 6 Life on Planet Earth ...................................................................................................... 6 Bioaccumulation .......................................................................................................... 7 Cycles of Matter in Ecosystems .................................................................................... 7 Energy in Ecosystems ................................................................................................... 8 Food Webs ................................................................................................................... 8 Ecological Pyramids ...................................................................................................... 9 Biomass and Fossil Fuels .............................................................................................. 9 Biotic and Abiotic Influences on Ecosystems .............................................................. 10 Symbiosis ................................................................................................................... 11 Ecological Succession ................................................................................................. 11 Importance of Bio-­‐Diversity ....................................................................................... 12 Matter ....................................................................................................................... 13 Physical Changes of Matter ........................................................................................ 13 Types of Properties .................................................................................................... 14 Chemical and Physical Properties .................................................................................................................... 14 Qualitative and Quantitative Properties ....................................................................................................... 14 Conversion of Mass .................................................................................................... 14 Energy ........................................................................................................................ 15 WHMIS ...................................................................................................................... 15 MSDS ......................................................................................................................... 15 HHPS .......................................................................................................................... 15 The GUESSS Method .................................................................................................. 16 Mass and Volume ...................................................................................................... 16 Density ...................................................................................................................... 16 Buoyancy ................................................................................................................... 17 Particle Theory of Matter ........................................................................................... 17 Classification of Matter .............................................................................................. 17 Atomic Theory ........................................................................................................... 18 Dalton’s Atomic Theory – The Ball Model: 1800’s .................................................................................... 18 Thomson’s Discovery of Electrons (Cathode & Anode Experiment) ................................................ 18 Thomson’s Model .................................................................................................................................................... 18 Ernest Rutherford’s Gold Foil Experiment – 1911 ................................................................................... 18 Bohr Planetary Model – 1912 ............................................................................................................................ 19 Syed Kamran Isotopes ..................................................................................................................... 19 Naming Isotopes ...................................................................................................................................................... 19 Atomic Mass .............................................................................................................................................................. 19 Atomic Structure ........................................................................................................ 20 Atomic Number ........................................................................................................................................................ 20 Mass Number ............................................................................................................................................................ 20 Atomic Notation ....................................................................................................................................................... 20 Periodic Table of Elements ......................................................................................... 21 Mendeleev – Concept of Periodic Table ........................................................................................................ 21 The Current Periodic Table ................................................................................................................................. 21 Elements ...................................................................................................................................................................... 21 Valence Electrons .................................................................................................................................................... 21 Main Categories of the Periodic Table ........................................................................................................... 21 Groups and Periods ................................................................................................................................................ 22 Classes .......................................................................................................................................................................... 22 Ions ............................................................................................................................ 23 Introduction ............................................................................................................................................................... 23 Cations ......................................................................................................................................................................... 23 Anions .......................................................................................................................................................................... 23 Why does this occur? ........................................................................................................................................ 23 Naming Ions ............................................................................................................................................................... 23 Chemical Symbols ...................................................................................................... 24 Counting Atoms in Compounds ........................................................................................................................ 24 Combining Capacity ............................................................................................................................................... 24 Building a Molecule ................................................................................................................................................ 24 Naming Compounds ............................................................................................................................................... 25 Double and Triple Bonds ............................................................................................ 25 Drawing Diagrams ...................................................................................................... 25 Bohr-­‐Rutherford Diagram ................................................................................................................................... 25 Bohr-­‐Rutherford Ion Diagram ........................................................................................................................... 25 The Cell ...................................................................................................................... 26 Electric Current ........................................................................................................................................................ 26 Circuit ........................................................................................................................ 26 Components ............................................................................................................................................................... 26 Types of Circuit ........................................................................................................................................................ 27 Current ...................................................................................................................... 27 Voltage (Potential Difference) .................................................................................... 27 Power ........................................................................................................................ 27 Electrical Resistance ................................................................................................... 28 Loads ............................................................................................................................................................................. 28 Conductors ................................................................................................................................................................. 28 Wires ............................................................................................................................................................................. 28 Superconductors ..................................................................................................................................................... 28 Safety ........................................................................................................................ 29 Syed Kamran Circuit Breakers ....................................................................................................................................................... 29 Fuses ............................................................................................................................................................................. 29 Wall Outlets ............................................................................................................................................................... 29 GFCI ............................................................................................................................................................................... 29 Surge Protector ........................................................................................................................................................ 29 Human Conductivity and Resistance .......................................................................... 29 Resistance of human body .................................................................................................................................. 29 Direct and Alternating current ................................................................................... 30 Alternating Current ................................................................................................................................................ 30 Direct Current ........................................................................................................................................................... 30 Electricity flowing to your house ................................................................................ 30 Electrical Production .................................................................................................. 30 Non-­‐renewable Resources .................................................................................................................................. 30 Fossil Fuels .................................................................................................................................................................. 30 Nuclear ......................................................................................................................................................................... 31 Renewable Resources ........................................................................................................................................... 31 Solar .............................................................................................................................................................................. 31 Wind .............................................................................................................................................................................. 31 Hydro-­‐electric Electricity ..................................................................................................................................... 31 Tidal Energy ............................................................................................................................................................... 32 Electricity Production in Canada ...................................................................................................................... 32 The Future .................................................................................................................................................................. 32 Nuclear Fusion .......................................................................................................................................................... 32 Geo-­‐Thermal .............................................................................................................................................................. 32 Static Electricity ......................................................................................................... 32 Insulators .................................................................................................................................................................... 32 Conductors ................................................................................................................................................................. 32 Laws of Electric Charges ............................................................................................. 33 Electrostatic Series ..................................................................................................... 33 Charging ..................................................................................................................... 33 Friction ........................................................................................................................................................................ 33 Contact ......................................................................................................................................................................... 33 Induction ..................................................................................................................................................................... 33 Semiconductors ......................................................................................................... 34 Grounding .................................................................................................................. 34 Lighting ...................................................................................................................... 34 Circuit Diagrams ......................................................................................................... 34 The Universe .............................................................................................................. 35 Distance in Space ....................................................................................................... 35 Astronomical Unit (AU) ........................................................................................................................................ 35 Light Year .................................................................................................................................................................... 35 Galaxies ..................................................................................................................... 35 Syed Kamran Spiral ............................................................................................................................................................................. 35 Elliptical ....................................................................................................................................................................... 35 Irregular Galaxies .................................................................................................................................................... 36 Constellations ............................................................................................................ 36 Our Solar System ....................................................................................................... 36 Sun ................................................................................................................................................................................. 36 Mercury ....................................................................................................................................................................... 36 Venus ............................................................................................................................................................................ 36 Earth ............................................................................................................................................................................. 36 Mars .............................................................................................................................................................................. 36 Jupiter ........................................................................................................................................................................... 36 Saturn ........................................................................................................................................................................... 37 Uranus .......................................................................................................................................................................... 37 Neptune ....................................................................................................................................................................... 37 Poor old Pluto ........................................................................................................................................................... 37 Best of wishes on the Exam :D!! ................................................................................. 37 Syed Kamran Ecology Notes Introducing Ecosystems Ecosystem: All the living and non-­‐living organisms of a certain region or area interacting. Example of ecosystems include: Forests, Swamps, and Coral Reefs. Living Components of an ecosystem are called Biotic and non-­‐living are abiotic. Population: All the individuals of a single species in a certain region or area make a population. Community: Individuals from all populations of a certain region or are form the community. This does not include abiotic factors. Characteristics of an Ecosystem: Organisms: Organisms vary from ecosystem to ecosystem. Temperature Range: The weather patterns of each ecosystem, depicts what organisms live there. Precipitation: The amount of rain and snow fall controls the climate of an ecosystem. Sustainability: Sustainability is the ability to maintain ecological balance; today most ecosystems are sustainable. This means that their characteristics will remain the same over a long period of time. Human actions can disturb the biotic and abiotic factors of an ecosystem, thus reducing its sustainability. Some actions can damage an ecosystem to a point where it is no longer sustainable. E.g. Oil Spills. Man-­‐Made Ecosystems: Most ecosystems are not mad made, however ecosystems such as parks are mad made. These ecosystems require constant management and are usually not sustainable. i.e. If you left a farm for three years would it look the same when you came back. Life on Planet Earth The Spheres of Earth: The earth has three spheres surrounding it. Unlike the moon, the Earth’s gravity is strong enough to hold gases close to the surface. • • • Atmosphere: Layer of gases extending upward for hundreds of kilometres, and consists of 78% nitrogen (N2), 21% oxygen (O2) and the remaining 1% is argon, water vapour, CO2, etc. This is crucial to life on Earth, and acts like a blanket providing moderate temperatures. Lithosphere: The rocky outer shell of Earth containing rocks and minerals. It is 50-­‐ 150 km in thickness. Hydrosphere: All the water on the Earth (above and below the surface). This includes all oceans, lakes, ice, clouds, etc. The Biosphere: All the life forms that exist within all the three spheres of Earth (Atmosphere, Lithosphere, Hydrosphere) Syed Kamran Bioaccumulation Bioaccumulation: A process in which materials, especially toxins, are ingested by an organism at a rate greater than they are eliminated. Biomagnifications: The process, in an ecosystem, in which a higher concentration of a substance in an organism is obtained higher up the food chain. Cycles of Matter in Ecosystems Biogeochemical Cycles: The movement of matter through biotic and abiotic factors. Matter cannot be made or destroyed. Nutrients are produced from substances already in the environment. They are four main biogeochemical cycles: 1. The Water Cycle: 2. The Carbon Cycle: Carbon is cycled through the lithosphere, atmosphere, hydrosphere, and biosphere. Carbon is an important element, it is the basic building block of living things. Carbon is recycled by photosynthesis and respiration. 3. The Nitrogen Cycle: The series of processes in which nitrogen compounds are moved through and abiotic environment. Nitrogen is taken from the atmosphere by soil bacteria by nitrogen fixation – a process that converts nitrogen gas into nitrogen containing compounds. Nitrogen is than available to producers, which animals eat. Decomposers feed on dead animals, while Denitrifying bacteria release the nitrogen back into the atmosphere. 4. The Phosphorus Cycle: Phosphorus starts in rocks, which break down into soil. Plants use the phosphorus in the soil; the plants are eaten by animals, which are decomposed by bacteria, which release the phosphorus back into the soil. Syed Kamran Energy in Ecosystems Types of Energy: • • • Radiant Energy: Energy that travels through empty space. o 70% of radiant energy from the Sun is absorbed by the hydrosphere and lithosphere, and converted into Heat. § 51% Absorbed by land and oceans § 19% Absorbed by Atmosphere and Clouds o 30% of the radiant energy is reflected back into space. Light Energy: Visible forms of radiant energy Thermal Energy: The form of energy transferred during heating and cooling (warms the atmosphere, evaporates water, produces winds) Photosynthesis: The process in which the Sun’s energy is converted into chemical energy (Glucose/Sugar). This occurs only in producers, an organism that makes its own energy rich food compounds using the Sun’s energy. Plants use chlorophyll to capture light energy. The formula of photosynthesis is !"#$%& !"#$"!% + !"#$% !"#!! !"!#$% !"#$% + !"#$%&. In oceans algae and cyan bacteria use chlorophyll to capture light energy for photosynthesis. Cellular Respiration: The process by which sugar is converted into carbon dioxide, water and energy. Organism use released energy for any of the activities carried out by its cell. The formula of cellular respiration is !"#$% + !"#$%& !"#$%& !"#$"!% + !"#$% + !"!#$%. Food Webs Ecological Niches: An ecological niche is simply the function of a species serves in its ecosystem; thus no two species have the same ecological niche. Producers vs. Consumers • • Producers: These are always plants that harness the sun’s energy with chlorophyll. Consumers: Living things that eat producers in addition to other consumers. o Herbivore: Animals that eat only plants (producers) o Carnivore: Animals that eat only meat (other consumers) o Omnivore: Animals that eat both plants and meat (producers and consumers) o Scavenger: Animals that feed on the remains of another organisms. Food Chain: A sequence of organisms, each feeding on the next, displaying how energy is following from one organism to the next. E.g. Pine cone àRed Squirrel àWeasel àGoshawk. Arrow head is pointing toward the consumer. These chains are not exhaustive and simply show feeding relationships. If one link of a food chain is broken it would result in numerous problems within the chain. Syed Kamran Trophic Level: The level of an organism in an ecosystem depending on its feeding postion. • • • • First Trophic Level: Producers e.g. Plants Second Trophic Level: Will eat producers only e.g. Small Animals (primary consumers) Third Trophic Level: Can eat primary consumers and producers e.g. Mid-­‐sized animals (secondary consumers) Fourth Trophic Level: Can eat primary, secondary consumers, and producers e.g. Large animals (tertiary consumers) Food Webs: A visual representation that much accurately displays who eat who within a community. It is usually highly complex as organisms feed upon several species. Similar to food chains the arrow points from the organism being eaten to the organisms that is eating it. Food webs are used to figure out what may happen when a species is removed or added to an ecosystem. Ecological Pyramids Ecological Pyramids: Pyramids that display the relationship between trophic levels in ecosystems. • • • Energy: Displays energy loss between trophic levels; only about 10% of the energy is passed on to organisms at the next trophic level. Only 10% of the energy is passed on because organisms use 90% of the energy for cellular respiration. The energy is released as heat and absorbed by the ecosystem. Numbers: The number of organisms that make up each tropic level. This pyramid are sometime bigger at the top. Biomass: Represents the mass (weight) of all the living organisms within that trophic level. Biomass and Fossil Fuels Biomass: Biological material from living, or previously living organisms. This material is usually recycled allowing the material to be reused. If biomass is trapped in places without oxygen, with is required to break down living matter, over time the trapped biomass is converted into fossil fuels. Burning Fossil Fuels: The burning of fossil fuels is the world’s fastest way of producing energy. Oxygen is used to produce energy and Carbon Dioxide. Fossil fuels have accumulated for millions of years, but in recent years human have burned significant portion of the Earth’s reserves. • Suspicion: Some believe that the CO2 being released is responsible for Greenhouse Effect. Syed Kamran Global Warming: The Greenhouse Effect is predicted to be causing Global Warming; this effect states rising of CO2 in the atmosphere is responsible for the increase in temperature. However, this has not been proven thus far. Fuel from Waste: Some bacteria are known to break down waste into sugar, through a process called fermentation. This released a gas called methane (CH4). This gas is then collected and burn to generated electricity. Acid Precipitation: Acids fall to the earth as a form of precipitation (rain, sleet or snow). • • • • When fossil fuels are burned they release undesirable substances Nitrogen Oxides and Sulfer Dioxide, are released and combine with water to form compound acids, such as Nitric Acid & Sulphuric Acid) Acid Precipitation damages many things: o Forest soils lose nutrients killing life. o When mixed with water in lakes and oceans, it damages the ecosystems and kills aquatic life. o In addition, acid also damages stone work. Efforts to reduce Acid Precipitation o Reduce burning of fossil fuels o Improve technologies to prevent Nitrogen Oxides and Sulfer Dioxide from escaping power plants. o Rising the standard for factories and motor vehicle emissions. pH: The measure of acidity and basicity of a substance. • • • pH 7: Neutral e.g. Pure Water and Blood pH below 7: Acidic e.g. Vinegar (pH:3) pH above 7: Basic e.g. Ammonia (pH:12) Biotic and Abiotic Influences on Ecosystems Limiting Factor: Any factor that places a limit on the size of a population, this can be biotic or abiotic. These factors can indirectly effect another population in the community. • • Biotic limiting factor: Amount of food available Abiotic limiting factor: Temperature, and availability of water. Tolerance: Ability of an organism to withstand many conditions (abiotic and biotic). If the organism is the optimal zone, it will perform the best and thrive. Carrying capacity: The number organisms of a single species that an area the support forever. Syed Kamran Symbiosis 1. Mutualism: Both species benefit from each other. 2. Commensalism: Both species benefit (Taken out of the curriculum):P 3. Parasitism: One species benefits at the expense of another species. This harms the host, however does not kill them. Predator – Prey Relationship: The predator-­‐prey relationship act to regulate the population of each species. e.g. If there are more predators the prey population will decrease, thus also bringing down the predator population because there is less to eat. − − Predator: Eats plants and animals. This are consumers that eat other organisms Prey: The organism that is eaten by the predator Population Factors: Factors which display how a population changes. • • • • Nataility (Births) Mortality (Deaths) Immigration (Animals coming in) Emigration (Animals leaving) Population Equation: !"# !"#$%&'(") = !"# !"!#$%&'"( + !"#"$%#& + !""#$%&'#() − !"#$%&'$( − !"#$%&'#() Exponential Growth: Rapid, uncharacteristic growth which occurs for a short period of time. Usually happens when a species is introduced into a new ecosystem that has lots of resources or when predators are removed. Ecological Succession Ecological Succession: Gradual changes, in the types of species that live in an area. The replacement of one plant community by another through natural processes over time. This process takes over 100 years. • • • Primary Succession: Begins in a place without soil, due to flooding. The species that are first to arrive are those who do not need soil to survive. These are called Pioneer Species. These species help break down rocks into smaller pieces to produce soil; when they decompose they also add organic matter to the rock to make soil. As the small species die off more plants slowly begin to grow. Primary Succession would look similar to the following: Dune grasses à Cottonwoodsà Shrubsà Oaks à Beeches Maples. As more plants begin to grow animals also begin to arrive . Secondary Succession: Starts in an area where soil is already present, this usually occurs after a forest fire. Unlike Primary Succession, soil is already present so there is no need of Pioneer Species. Pond Succession: Organic substances will build up at the bottoms of ponds and lakes and convert the pond into marsh and later into dry land. An example of Pond Succession is: Pond à Marsh à Dry Land/Grasses à Shrubs à Forest. Syed Kamran Terminology: • • Pioneer Species: The first species to start the process of succession. Climax Community: The last or final stage in succession e.g. Forest is matured. Importance of Bio-­‐Diversity Biodiversity: The amount of life in a particular ecosystem, this is measured by counting all the species and is referred to as Species Richness. i.e. Tropical rainforest have the highest biodiversity of any ecosystem. Why humans value biodiversity: • • • • Different trees clean the air we breathe Animals provide, we require different tasting animals J Stabilizes and moderates the climate Benefits our industries such as forestry, farming, and fishing At Risk Species: • • • • • Vulnerable: Any species that is at risk because of declining numbers in population Threatened: Any species that is likely to become endangered if factor that make it vulnerable are not reversed. Extirpated: The species no longer exists in one of its previous habitats Endangered: The species is very close to extinction in a large area. Extinct: The species cannot be located anywhere in the world. Keystone Species: Species that are crucial for the health or survival of other species e.g. Bats. These species are hard to identify until an ecosystem fails due to their absence. Natural Causes of Extinction: Extinction of species that have not been blamed on humans. • • • • Competition with other organisms Environmental disasters Climate change Low Reproduction Human Cause of Extinction: Extinction of species which is blamed upon humans. As human population increases, the amount of species remaining declines. • • • Invasion of habitat Over Hunting Pollution Syed Kamran Chemistry Notes Matter Matter is everything; it is around us, above us and below us. We ourselves are made up of matter. It is defined as any substance that has both mass and volume. Matter is classified into three states, which are as following: Solids: The molecules are close together and can only move a little bit. They have a definite shape and volume, in addition are not compressible. • Liquids: The molecules are close together, but are free to move. Liquids have the ability to change shape, but have a definite volume. You can compress liquids a little bit. • Gases: The molecules are far apart from each other, and are free to move as they wish. Gas has no particular shape, and can and no definite volume. Gases are also compressible. Note: Light, heat, and types of energy are not forms of matter. • Physical Changes of Matter There are certain processes that matter undergoes to change physical state. • • • • • • Ice to Gas: Sublimation Gas to Ice: Sublimation Liquid to Gas: Evaporation Gas to Liquid: Condensation Liquid to Solid: Solidification Solid to Liquid: Liquefaction Syed Kamran Types of Properties Properties of substances are not exclusive to one list, and they overlap. i.e. A chemical property can also be a qualitative property. Chemical and Physical Properties Chemical Properties: A characteristic is deemed a chemical property if it reacts with another substance to create a new substance. Physical Properties: A characteristic is deemed a physical property if it does not produce a new substance. Some examples of physical properties that you should know for the test are as follows: • • • • • • • • Hardness: Measure of resistance of a solid being scratched Ductility: Ability to pulled into wires Malleability: Ability to be hammered into thin sheets Crystal Form: Solid form of minerals where you see a definite structure of cube or blocks with a regular pattern or natural shape Viscosity: How easily a liquid moves Colour: The hue of light related of a substance Solubitlity: Ability to dissolve in another substance, such as water Density: Amount of matter per unit area. Qualitative and Quantitative Properties Quantitative: Properties that are based on a number or measurement Qualitative: Properties that are descriptive properties. Conversion of Mass A principal in science is that, matter cannot be made or destroyed during a chemical change. Consider the following example: Butane + Oxygen → Carbon dioxide + Water 58 g 208 g 176 g 90 g 266 g 266 g Syed Kamran Energy The ability to do to work and this comes in many different forms: • • • • • • • Heat Mechanical o Kinetic o Potential Electrical Sound Chemical Nuclear Electromagnetic WHMIS WHMIS stands for Workplace Hazardous Material Information System. It is used to identify, classify products, and train and educate workers. There are eight classes of Hazard Symbols. Which are as follows: • • • • • • • • Class A: Compressed Gas Class B: Flammable and Combustible Material Class C: Oxidizing Material Class D1: Materials Causing Immediate and Serious Toxic Effects Class D2: Materials Causing other Toxic Effects Class D3: Biohazardous Infectious Material Class E: Corrosive Material Class F: Dangerously Reactive Material MSDS MSDS stands for Material Safety Data Sheet, and are available for all used chemicals and contain important information. HHPS Hazardous Household Product Symbols are found on all potentially dangerous household products. It indicates both the type of danger and the degree of risk. Syed Kamran The GUESSS Method This is kind of important and might be on the test, but anyways: G: Given U: Unknown E: Equation S: Substitute S: Solve S: Statement Mass and Volume Mass: Mass is a physical property that displays the amount of matter and object contains. It is measured in grams and kilograms. Volume: A physical property that represents how much space an object occupies. It is measured in milliliters, liters, centimeters cubed. Density Density is a quantitative property of matter, which describes how much mass per unit volume a substance occupies. Basically, it’s the amount of mass in a specific amount of space. Density is measured in grams/mL Formula for Density: Density = Mass/ Volume In order to find the density of a submerged object under water we use the formula: % !"#$%&'%( (!"#$% !"#$"%) ! !"#$%&' !" !ℎ! !"#$% Density of object % submerged (in decimal) Mass Density of Fluid ** Remember the triangle for the formulas. Density Volume Syed Kamran Buoyancy Buoyancy is the upward force upon an object when it is submerged in water. This is why you weigh less in water than on land, because buoyancy pushes you upward. • • • Negatively Buoyant: Will sink Positively Buoyant: Will float Neutral Buoyant: Will neither sink or float, it will hang in the middle. Water has the density of 1g/mL, meaning anything that has a density of less than 1g/mL will float in water. Particle Theory of Matter The particle theory of matter covers basic laws: 1. All matter is composed of particles 2. The particles of one substance are the same. Different substances are made up of different particles. 3. There are attractive forces between particles. 4. Particles are always moving, except for absolute zero J 5. The more energy particles have, the faster they move and the higher their temperature. ** Temperature is a measure of speed of the particles. Classification of Matter All matter in the universe can be classified into two basic categories.: 1. Mixtures: Consists of two or more substances mixed together, not chemically bonded. a. Homogeneous: The mixture has one phase and has identical properties throughout. b. Heterogeneous: The mixture has more than one phase, and does not look the same throughout the object. 2. Pure Substances: Matter that consists of one substance, chemically bonded or alone. a. Elements: Any element on the periodic table of elements b. Compounds: Two or more elements chemically bonded. Syed Kamran Atomic Theory To generalize the size of an atom, lets take the Size of Earth : Soda Can = Soda Can : Atom. All elements have atoms; they are the smallest particles that still carry properties of their elements. It is possible to view an atom under scanning electron microscopes. The Greek philosopher Democritus (460 B.C – 370 B.C) was the first to suggest the existence of atoms. As time progressed so did the Atomic Theory. Dalton’s Atomic Theory – The Ball Model: 1800’s • • • Dalton referred to the atom as a small, hard, indestructible sphere that cannot be subdivided. Atoms of different elements have different properties. The atom is the smallest particle of any element. Thomson’s Discovery of Electrons (Cathode & Anode Experiment) • • • • When there are low gas pressures, a ray is emitted from the negatively charged cathode. The ray then moves to the positively charged anode. Thomson suggested the rays were made up of negatively charged particles found in the atoms, and later said that all atoms have negatively charged particles. These are now called electrons (e-­‐) Thomson’s Model • • Thomson believed that atoms did not have any charge, and since his particles were negatively charged then there also must be positively charged particles within the atom. Thomson concluded that electrons were embedded like raisins in a positively charged “Bun”, thus its was called the “Raisin Bun” model. Ernest Rutherford’s Gold Foil Experiment – 1911 • • • Alpha particles (+ charged) – The alpha particles were fired at a thin sheet of gold foil, and particles that hit on the detecting screen (film) are recorded. Rutherford expected that the particles would go through but some might be diverted, however some particles collided and rebounded off something very dense. Rutherford concluded that most of the atom is empty space, it contains a small dense, positively charged nucleus at the center, and has negatively charged electrons that revolve around the nucleus. Syed Kamran Bohr Planetary Model – 1912 • • In 1912, Neils Bohr thought that if the electrons were negatively charged and the nucleus positively, why don’t they attract and collide. (Opposites attract). By passing different amounts of current through hydrogen gas, Bohr observed that different coloured light were given off. He concluded that electrons could gain energy by absorbing light (quantum). Isotopes Dalton believed that all atoms of an element were the same (identical), but that was later discovered to be incorrect. Atoms of the same element can have different number of neutrons. Frederick Soddy proposed the idea of isotopes in 1912. Isotopes are atoms of the same element having different masses, due to varying number of neutrons. Naming Isotopes When naming isotopes we put the mass number after the name of the element. • • • Carbon – 12 = carbon with an atomic mass of 12 (6p+, 6no) Carbon – 14 = carbon with an atomic mass of 14 (6p+, 8no) Note the number of protons does not change only the number of neutrons. Atomic Mass When calculating the atomic mass of an element, we are actually concerned about the average atomic mass of all its kinds of atoms. This is measured in Atomic Mass Unit (amu). To calculate the atomic mass of an element, we need the atomic mass of all its different atoms and their percentage of abundance. Look at the following example: • Isotope 10X: • Isotope 11X: • Element X: mass = 10.012 amu relative abundance = 19.91% = 0.1991 mass = 11.009 amu relative abundance = 80.09 % = 0.8009 10.012 × 0.1991 = 1.993 !"# (Isotope 10X’s mass times abundance) 10.012 × 0.8009 = 8.817 !"# (Isotope 11X’s mass times abundance) !"#$%& !"## !" !"#$#%& ! = 1.993 + 8.817 = 10.810 To watch a cool atom video: http://www.youtube.com/watch?v=0kUSqHYcF8g (My Science ISU video). Syed Kamran Atomic Structure Atoms are the building blocks of all matter; they are composed of the smaller bits, as follows: Proton: The proton has a positive charge has a mass of one and is inside the nucleus. The number of protons determines the atomic number. Electron: The electron has a negative charge has a mass of zero and is outside the nucleus. The number of electrons determines the ion. Neutron: The neutron has no charge has a mass of one and is inside the nucleus. The number of neutrons determines the isotopes of the element. • • • Atomic Number Atoms of different elements differ from one another because they contain different number of protons. The atomic number of an element is the number of protons in the nucleus. In addition when an atom is in normal form, the number of protons is equal to the number of electrons. Mass Number The atomic mass of an element is determined by the sum of the number of neutrons and number of protons. This refers to the weight of the atom and is measured in Atomic Mass Unit (amu). There are 6 × 10!" or 600,000,000,000,000,000,000,000 amu’s in one gram. Atomic Notation The chemical symbol tells us what atom it is; this is usually short form however sometimes it isn’t because they were named in Latin. The first letter is always a Capital Letter and is followed by a lower case letter. Syed Kamran Periodic Table of Elements The periodic table of element is the most useful tool to chemists, and you in the test. It has a ton on information in regards to all the known elements. The periodic table organizes elements in a particular so that you can determine physical and chemical properties for an element from its location on the table. You can also predict what the element will react with chemically. * Note: You are given a periodic table of elements before the test starts. Mendeleev – Concept of Periodic Table In 1869, Dmitri Ivanovitch Mendeleev created the first accepted version of the periodic table of elements. He grouped the elements according to their atomic mass, as he did he found that the families had similar chemical properties. Blank spaces were left open to add the new elements. The Current Periodic Table Mendeleev was not that far off the current periodic table, today the elements are put in rows by increasing ATOMIC NUMBER. The horizontal rows are called periods and are labeled from 1 to 7. The vertical columns are called groups are labeled from 1 to 18. Elements Scientists have identified 92 naturally occurring elements and created about 26 others. The elements alone or in combinations, make up our bodies, our world, our sun, and in fact, the entire universe. Valence Electrons Valence electrons are the number of electrons at the outer energy level of an atom. These are the electrons that are used when atoms bond together. Main Categories of the Periodic Table Metals: Metals are good conductors of heat and electricity. They are shiny, ductile, and malleable. Metal corrode in water. Non-­‐Metals: Non-­‐metals are poor conductors of heat and electricity. They are not ductile, and malleable. Solid non-­‐metals are brittle, break easily and are dull. Many non-­‐metals are gases. Metalloids: Metalloids have properties of both metals and non-­‐metals. They can be shiny or dull. They can conduct heat and electricity, however not as well as metals. They are also ductile, and malleable. Syed Kamran Groups and Periods Within the Periodic table of elements, elements are also organized via groups and periods. Groups (families): Groups are the vertical columns that have similar properties. Some of the columns have been given special names to distinguish them. All elements in a family have the same number of valence electrons. Periods: Periods are the horizontal rows that do not have similar properties. Sizes of atoms decrease as we move left to right across a period; this is due to the increasing number of protons in the nucleus, resulting in a stronger electrical attraction between the nucleus and electrons. In addition the electronegativity increase as you move left to right across a period. The first element in a period is always an active solid, and the last is always an inactive gas. Groups (families) Periods Classes There are many classes within the Periodic table of elements; most of the elements have similar properties. Hydrogen: The hydrogen square sits atop of Group 1, but it is not a member of that family. It is a gas at room temperature. It has one proton and one electron in its electron level. Alkali Metals: The alkali family is found in the first column of the periodic table. Atoms of the alkali metals have a single valence electron. They are shiny, have the consistency of clay, and are easily cut with a knife. They are the most reactive metals, and react violently with water. Alkali metals are never found as free elements in nature, however always bonded with another element. Alkaline Earth Metals: Have two valence electrons and are never found uncombined in nature. Transition Metals: They are good conductors of heat and electricity. Can chemically combine with oxygen to form compounds. Syed Kamran Halogen Family: Halogens have seven valence electrons, why they are the most active. They are never found free in nature. Noble Gases: Noble gases are colorless and extremely unreactive. They outer most energy level is full, and due to this they are called inert. They are found in small amounts in earth’s atmosphere. Rare Earth Elements: The thirty rare earth elements are composed of the lanthanide and actinide series. Most of these elements are synthetic or man-­‐ made. Ions Introduction Atoms are made up of particles, which either have a positive charge or negative charge. The positive or negative charges cancel each other out, so the net charge in an atom is zero. The positively charged particle is called a proton, and the negatively charged particle is called the electron. In atoms the protons are in the center and the electrons are on the outside, only electrons can be removed from atoms. If you add electrons to an atoms in becomes negatively charged, and if you remove them they become positively charged. An atom with a positive or negative charge is called an ION. Cations Cations are ions with a positive charge. If an atom loses an electron or more it is called cation. Anions Anions are ions with a negative charge. If an atom gains an electron or more it is called an anion. Why does this occur? All atoms want to become stable, and in order to become stable there outer most energy level must be complete. To do this they can either lose an electron and become a cation or gain an electron and become anion. The atom will do what ever is easier, in other words it would prefer losing an electron instead on gaining seven in the following case. * Note: We only work with groups 1, 2,13,15,16, and 17 when dealing with ions. Naming Ions Positive Ions: The name is the same of the element followed by ion. E.g. Na+ = Sodium ion Negative Ions: The name is determined by removing the end and adding ide. E.g. O-­‐ = Oxide ion Syed Kamran Chemical Symbols All the elements on the periodic table of elements have a single symbol, which is made up of 1 or 2 letters. Just as a single symbol is used to represent a single element, multiple symbols can used to represent compounds. Counting Atoms in Compounds Most of the elements can be combined in many different ways to make compounds. E.g. NaHCO3 is composed of Na, H, C, and O. In order to show the number of atoms in a compound we use subscripts. In H2O, we have two Hydrogen and one Oxygen atom. If there is only one atom of an element one is not written in the subscript. If a number is put in front of a compound like, 2H20, you must multiply that number by the each element, so 2H20 is equal to H20 + H20. In which case there are four Hydrogen atoms, and two Oxygen atoms. Combining Capacity Atoms can only make a specific number of connections with other atoms. The number of connections an atom can make is called the combining capacity. When atoms combine, it is called a bond. Building a Molecule Lets take two elements, Hydrogen and Oxygen, if we want to want to bond them we need to know their combining capacity. Hydrogen: • Combining capacity: 1 • Combining capacity: 2 Oxygen: We simply switch the combining capacities and write them as subscripts for the other element. So we write H20. 1 is not written in subscripts. The above is a structural diagram. In order to satisfy a compound you need to make the right number of connections. * Note: You will be given a list of common combining capacities for the test. Syed Kamran Naming Compounds Rules: 1. Metals combine with nonmetals in many compounds a. Metals will be on the left, when ever in doubt the one of the left goes first 2. Write the name of the metal first then the nonmetal 3. Change the ending of the nonmetal to “ide” E.g. Aluminum (metal) and Oxygen (nonmetal) Aluminum Oxide Double and Triple Bonds Sometimes atoms make more than one bond another atom, this can either be a double or triple bond. Drawing Diagrams There are a couple of diagrams you need to be able to draw for the test. They are as follows. Bohr-­‐Rutherford Diagram Lets take an element, with an atomic number less than 20. Oxygen • • • Eight Protons Seven Neutrons Eight Electrons Bohr-­‐Rutherford Ion Diagram Lets take an element, with an atomics number less than 20. Oxide (II) ion • • • Eight Protons Seven Neutrons Ten Electrons Syed Kamran Electricity Notes The Cell A cell stores chemical energy and transfers this energy as electrical energy. Two cells are connected to one another to make a battery. The cell’s chemical energy is used to move current around a circuit. Electric Current The cell is responsible to produce an electric current; the current is the flow of electrons from the negative terminal of the battery back to the positive terminal. Current is defined as the flow of electrons passing through a point. Circuit When circuit is closed, it means that there is a continuous path of metal connecting the positive and negative ends of the cell together, in which case all components will light up. However if there is a break in the circuit, it is known as an open circuit where the current cannot flow from the negative side to the positive. Components There are many components within circuit that you will encounter when drawing electrical diagram. 1. Cell: A cell is the power source of a circuit. Two cells make a battery. 2. Switch: A switch breaks a path within a circuit. 3. Lamp: A lamp or bulb is a component within a circuit. 4. Wires: The wire is the transport medium of electrons. 5. Fuses: A fuse is a moderator, which burns out if more than a certain amount of electricity passes through it. 6. Resistor: A resistor is component within a circuit, which restricts the flow of electrons. 7. Ammeter: An amp meter measures the current a circuit. It has to be connecter in series. 8. Voltmeter: A voltmeter measures the potential difference in a circuit. Syed Kamran Types of Circuit There are two types of circuits that you are going to be on test: 1. Series Circuit: The components are connected end to end, one after an other. i.e. They make a simple loop for the current to flow. If any the components blow all the whole circuit will not work. 2. Parallel Circuit: The components are side by side. This way the current is split to take different paths to reach each component. This way if one component blows the other will stay lit up. Current Current is the flow of electricity around a circuit, this runs from negative to positive. It is abbreviated to (I), however measured in Amps (A). Current is not measured in the number of electrons, however group of electrons, which are called coulombs. One coulomb is equal to 6.25 × 1018 electrons. One coulomb is equal to one ampere. In order to measure current you need an ammeter, which has to be connected in series. Within a series circuit the current is same all around, however in a parallel circuit the current is shared between all paths. Voltage (Potential Difference) The amount of energy each electron has within a circuit is called potential difference (Voltage). It is called potential difference cause it has the potential to do work. When an electron passes through a light bulb it enters with certain amount of energy, and when it leaves it has less, because the energy is used by the light bulb. Potential difference is the amount of energy lost from one point and another, so in order to calculate it we have to connect a voltmeter in parallel. Within a series circuit voltage is shared between all components, however is a parallel circuit voltage is same at all parts. Power Power is measured in Watts, and is determined by multiplying the Voltage and the Amperage. Electrical energy is measured by multiplying the number of watts by time. *Remember the following formula: Watts Voltage Amps Syed Kamran Electrical Resistance Resistance is the inability of electrical current to pass through a substance. When electrical current is resisted it is usually converted into heat, light, sound, or other types of energy. Some substances resist the flow of electrons, such as light bulb filaments. Light bulb filament take energy from the electrons and convert it into light energy. The symbol for resistance is Ω and it is measured in ohms. Resistance is in direct relation with current, the more resistance the lower the current. Loads Conductors A resistor or any device that transforms electrical energy into heat, motion, sound, or light. A substance that carries electrical energy without much or any resistance is known as a conductor. Conductors are usually used in wires, to allow transfer of electricity. Wires Resistance in wires is determined by the following factors: 1. 2. 3. 4. The type of material The length (greater the length the greater the resistance) The diameter (greater the diameter the lower the resistance) The temperature (hotter wires have greater resistance) Superconductors When electric current can flow through a substance with zero resistance, it is called a superconductor. This can be done by cooling a wire to absolute zero, which increase the efficiency of the wire as no energy is lost. *Remember the following formulas: Voltage Current Resistance Series: • IT= I1= I2 = I3 • VT= V1+ V2 + V3 • RT= R1+ R2 + R3 Parallel • IT= I1+ I2 + I3 • VT= V1= V2 = V3 • RT< R1 RT < R2 RT<R3 • Syed Kamran Safety In order to prevent fires from occurring within houses or apartment buildings several safety features have been installed to shutdown the electricity system from allowing too much electricity to flow. Circuit Breakers Circuit Breakers are a newer method of electricity control in many houses. They trip if too much electricity is flowing through the circuit, although you simply have to flip the switch to reset them. Fuses Used in older homes, the fuses work similar to the Circuit breakers except they melt the wire inside. In order to reset a fuse and allow electricity to flow you need to replace the fuse. Wall Outlets GFCI Wall outlets are made out of plastic primarily because plastic does not conduct electricity. In addition there are polarized plugs, requiring the person to but the plug in the right way, and lastly the have a ground hole for additional electricity. GFCI also known, as Ground Fault Circuit Interrupter is a device used breaks the circuit if it detects that the current between the energized and the neutral conductor is not balanced. (Meaning the current is flowing else were. It is usually put in bathrooms.) Surge Protector A surge protector is a device used to protect appliances from voltage spikes. The surge protector regulates voltage by either blocking or shorting voltages above a certain threshold. Human Conductivity and Resistance Humans can be electrocuted, however the voltage required to electrocute someone is dependent upon the current through the body, the resistance and the duration of the current. If 0.002 amps passes through your body your heart is disturbed, and if 0.02 amps passes through you, you will die. Resistance of human body • • Dry conditions: 100,000 Ohms Wet or broken skin: 1,000 – 5,000 Ohms High voltages can break down skin, reducing resistance to 500 ohms. Skin breaks down above 240 V Syed Kamran Direct and Alternating current Direct and Alternating current is the two methods of transferring electricity through a wire. Each has it advantages and disadvantages. Alternating Current Alternating current is when electricity when electrons hit one another passing energy along. This allows for higher voltage to be transferred over long distances, has less copper loss than DC, and is cheaper to produce. Direct Current Direct current is when electricity is moving in one constant direction; this is the type of electricity you find in batteries with constant positive and negative terminals. Electricity flowing to your house Coming from the hydro pole are three wires, two black and one white. The two black wires carry a voltage of 120 V, while the white wire is natural which is a way for the electricity to go back. The wires coming from the poles go to the electric meter outside your house, which is calculates the amount of electricity you use. From the electric meter the wires go to the electrical distribution panel located in your basement. Electrical Production There are two main sources of electricity production; they are Renewable or Non Renewable. Renewable sources renew themself over the course of a life span, while non-­‐renewable is not able to replenish itself within a lifetime. Non-­‐renewable Resources Non-­‐renewable resources include fossil fuels, such as oil, coal and gas. Energy is released from the combustion of these substances, and this is relatively cheap. However the world supply is decreasing of fossil fuels. Fossil Fuels Coal, oil and natural gas are called, “fossil fuels”. This is because they are formed from the remains of dead plants and animals. 1. Fossil Fuels are burned which heats water and produces steam 2. Steam causes turbine to spin. 3. Which causes generator to create electricity. Syed Kamran Nuclear Nuclear energy runs from the power of Nuclear Fission, which is a nuclear reaction. In this reaction uranium atoms are split releasing atomic energy. This has radioactive waste problems, and waste materials, which are deadly to human beings. Reactors use Uranium rods as fuel, and generate heat from nuclear fission. Nuclear fission is accomplished by a slow neutron hitting a uranium atom, which is split in half and releases energy. A seven gram fuel pallet produces the same amount of energy as 807 kg of coal, 667 L of oil, and 476 m3 of natural gas. 1. Fission makes heat. 2. Heat water to make steam. 3. Steam turn turbine. 4. Turbine turns generator. 5. Generator makes electricity. Renewable Resources Renewable resources are resources that can replenish themselves within one’s lifetime. Solar Solar energy is radiated energy from the sun, it is used for heating, and producing some electricity. Photovoltaic cells are used to directly convert sunlight into electricity. This power can be stored in a battery, which can allow for power later on. Solar water heating is used to heat water in glass panels, under direct exposure to the sun. Wind The rising of hot air and the falling of cold air is the cause of wind; this energy can be converted into mechanical energy and finally electrical energy by the use of windmills. Open flat fields are excellent areas for windmills. Hydro-­‐electric Electricity Water cycle causes precipitation to areas of high altitude, from which the water makes it back to the sea. When the water is falling down it is converted into mechanical energy and then electrical. Sometimes dams are built to control water flow and allow from fast speeds of water to turn the blades of the turbine, which are driving the generators. Syed Kamran Tidal Energy In tidal energy the motion of the tides is harnessed. This is caused by the moon’s gravity pulling up on the ocean’s water. It is similar to hydraulic. The only modern tidal energy generation station is in Nova Scotia. Electricity Production in Canada In Canada our electricity is produced by Fossil Fuels, Nuclear Energy and Hydroelectricity. We do not harness enough solar or wind power in this country. Energy Source Fossil Fuels Nuclear Energy Hydroelectricity Other Total % Supplied 26 % 50% 22% 2% 100% The Future The future survival of human beings is dependent upon the investing in new technologies such as Nuclear, and Geo-­‐Thermal. Nuclear Fusion This is the joining of two particles to form one large nucleus, nuclear fusion naturally occurs on stars such as the Sun. Geo-­‐Thermal This is thermal energy from below the earth’s crust, which can heat water into steam, which turns a turbine driving a generator. This can also be used for heating homes. Static Electricity Static electricity is the imbalance of positive and negative charges, when atoms lose electrons they become positively charged and when they gain electrons they become negatively charged. Electrons can be transferred (or stolen) from one object by the friction. (Refer to Charging). Insulators Conductors Objects or materials that hold their electron tightly are known as insulators. These objects do not allow objects to move through them. Objects or materials that have a lose hold on their electrons, and allow them to move easily from one atom to the next. Most metals are great conductors. Syed Kamran Laws of Electric Charges Similar to the laws of motion, electric charges have their own laws. The laws are as follows. 1. Opposite charges attract 2. Like charges repel 3. Positive and negative charges attract neutral objects (remember the balloon experiment) These laws are commonly exhibited in daily life such as a taking a wool hat off on a winter day. This results in your hair sticking up because electrons are transferred from your hair to the hat making them positively charged, your want to repel from one another and stand up. Electrostatic Series The electrostatic series is a table of arranged materials, which are ordered from least relative hold on electrons to greatest relative hold on electrons. There will be questions in regards to electrostatic series on the test. Charging There are three ways to charge objects to give them either a positive, negative or neutral charge. The three methods are as follows: Friction By rubbing to two objects together the one with less hold on its electrons will lose its electrons and become positively charged, while the one with a stronger hold on electrons will gain electrons and become negatively charged. Both surfaces obtain a different charge after being rubbed. Refer to the electrostatic series to determine what objects have a stronger hold on electrons. Contact Contact is the most common type of transfer, and the cause of static shocks. This occurs when two objects that have different charges touch each other and will transfer electrons to balance themselves evenly. Induction Induction is when an electric charge is transferred to an object without direct contact. Induction allows you to give objects a temporary charge by splitting them into two differently charged portions, or completely giving a charge by the use of a ground wire. If you bring a charged rod near a neutral object the opposite charge will face the rod, while the like charges will repel. This creates an induced object. If you attached a ground wire to an object and bring a charged rod near it, electrons will either flow out of the object or into the object, resulting in a completely charged object. Syed Kamran Semiconductors Semiconductors are non-­‐metals such as silicon; they allow electrons to move through them fairly well. Not as good as conductors and not as bad as insulators. Grounding Grounding is a process where you remove charges from an object and return it to neutral state. This process requires you to connect the object to the ground via a conductor. This is also called discharging. Grounding happens because the earth has an infinite supply of electrons and it doesn’t matter how many are added or stolen. The earth has 6.91 x 1096 electrons. When a positive object is grounded electrons are taken from the ground and added to the object, on the other hand if a negatively charged object is grounded, electrons will flow into the earth. This always results in the object ending up as neutral. Lighting Storm clouds form when cold and hot air meet. The masses of air churn together and lighting is created. Lighting strikes when negative charges in clouds are attracted to the positive charges in the ground. Lighting take the easiest path so it usually hits the highest object. Lighting strikes are unavoidable so people use light rods, which are connected to the ground. This allows all the lighting to be grounded. Circuit Diagrams You will have to solve circuit diagrams on the test so here is an example. R1 = 10Ω R2 = 35Ω R3 = 14 Ω V=9.0 V V = 4.5 V For resistor 1 the voltage is 4.5V, and the current is 0.45 A. For resistor 2, the current is 0.13 A. For resistor 3 the voltage is 4.5 V and the current is 0.32 A. If you have trouble understanding how to solve the circuit ask before the exam. Syed Kamran Space Quiz Notes The Universe The universe is basically everything; this includes all matter, all energy, and even things that are outside of Earth. The stud of things outside of Earth is referred to humans as astronomy. Distance in Space Distances in space are not measure in the same units as they are on Earth due to the fact that space is so big. If we were just to look at the distance between the Earth and the Sun it would be 150 million km, for two object that are relatively close in perspective of the whole universe. This is the reason scientists chose to make the AU or astronomical unit along with light year to measure distance within space. Astronomical Unit (AU) An astronomical unit is defined by the distance between the Earth and the Sun, which is 150 million km; so 1 AU is 150 million km. To find out how make AU’s a distance is, divide it by 150 million km. Light Year Due to that fact that distances in outer space are enormous, we use light years. When the Astronomical Unit (AU) becomes too small of a reference of measurement we use light years. A light year is basically travelling at the speed of light for one year. The speed of light is 300,000 km per second. A distance of one light year is equivalent to 9,460,800,000,000 km. Note: It’s practically impossible for anything except light itself to travel at light speed. Check out Einstein’s theory of relativity if you’re interested about speed of light. Galaxies A galaxy is system of stars and gases held together by a gravitational force, Edwin Hubble invented this system. Galaxies are classified in three main types: Spiral Spiral galaxies look like a pinwheel, similar to a large plate, with a bulge in the side. Some have many arms spiraling out from the center. Elliptical Range in shape from a either a perfect sphere to a weird stretched out ellipse. These contain the oldest stars in the universe and make up the large galaxies. Syed Kamran Irregular Galaxies These galaxies are the odd ones out, they do not really have a category. The range in shape and size and are made up of newly forming stars and old stars. Constellations Stars in the sky that form a shape or pattern are known as constellations. Constellations have been used for thousands of years for many purposes. Various constellations can be seen from different parts of Earth. Our Solar System Our solar system is comprised of the sun, eight planets, several moons and numerous comets and asteroids. Sun The sun is the largest object in our solar system; it is essentially a large ball of gas. It is comprised of 75% hydrogen, and 25% helium. The sun converts hydrogen into helium in its core, where the temperature is 15.6 million Kelvin, and the pressure is 250 billion atmospheres. Mercury Mercury has the shortest orbit around the sun, 0.24 earth years. Its mass is 0.05 times Earth, which makes it that 8th largest planet in our solar system. It is composed of a rock surface. Venus Venus has a orbit of 0.62 earth years, and its mass is 0.82 times Earth making it the 6th largest planet in the solar system. Venus is also the brightest planet. Earth Earth is the 3rd planet from the Sun. It has one moon which is 1/6 its mass. Earth takes 365.25 days to orbit around the Sun, and is the only planet currently known to inhabit living life forms. The Earth is on a tilted axis (23.5o) Mars Jupiter Mars is known as the red planet due to its iron compositions. It has the mass of 0.11 times Earth and takes 1.88 Earth years to orbit the sun. It also has solid carbon dioxide at its poles. Jupiter is 70% of the mass of the solar system outside the Sun. Its mass is 317.8 times Earth and it take 11.86 Earth years for it to orbit the Sun. It is not a solid and is composed of hydrogen and helium. Syed Kamran Saturn Similar to Jupiter, Saturn is made out of gas. It is known for its rings, which are made of ice. Saturn takes 29.46 Earth years to orbit the Sun and has the mass of 95.2 times Earth. Uranus Uranus is made out gas, and is the 4th largest planet in the solar system. It is flipped on its side and has smaller rings then Saturn. It take Uranus 84.01 Earth years to orbit the Sun, and its mass is 14.5 times Earth. The blue colour is from methane gas within its atmosphere. Neptune The last planet in the solar system, and is composed of gas. It is similar to Uranus however is a slightly darker blue. Its rings are sometimes visible. Neptune takes 164.8 years to complete one orbit and its mass is 17.1 times Earth. Poor old Pluto Pluto is no longer a planet as it is too small. There are many other dwarf planets similar to Pluto within our solar system. Pluto is smaller than the moon. Best of wishes on the Exam :D!!