Aromatic Iodination
advertisement
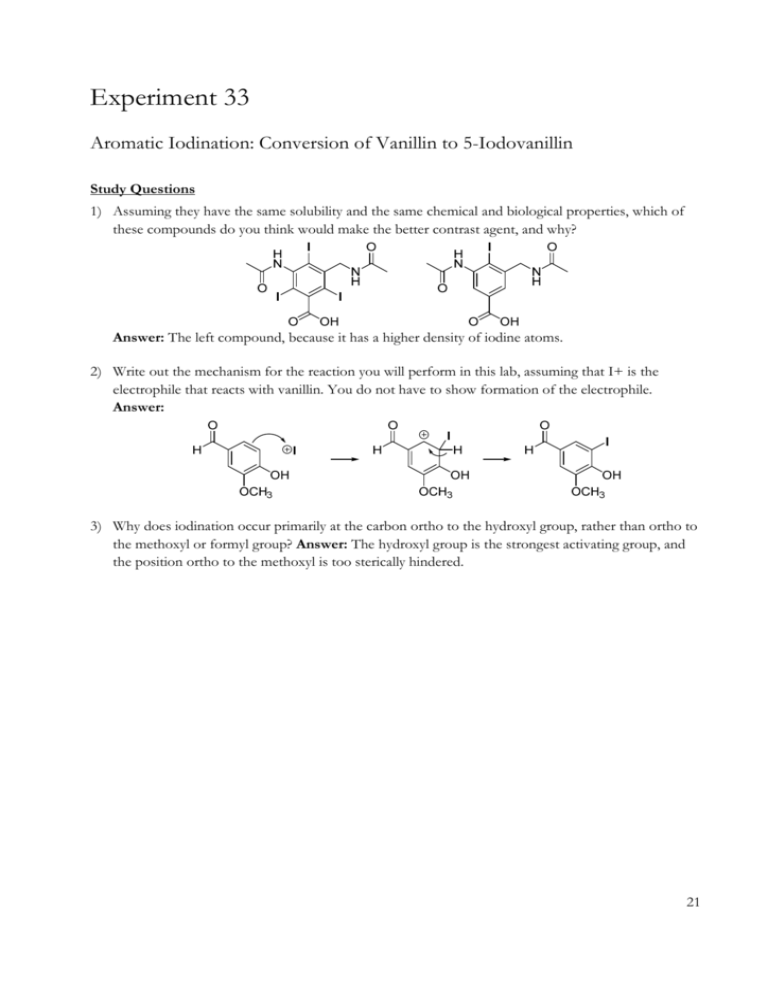
Experiment 33 Aromatic Iodination: Conversion of Vanillin to 5-Iodovanillin Study Questions 1) Assuming they have the same solubility and the same chemical and biological properties, which of these compounds do you think would make the better contrast agent, and why? Answer: The left compound, because it has a higher density of iodine atoms. 2) Write out the mechanism for the reaction you will perform in this lab, assuming that I+ is the electrophile that reacts with vanillin. You do not have to show formation of the electrophile. Answer: 3) Why does iodination occur primarily at the carbon ortho to the hydroxyl group, rather than ortho to the methoxyl or formyl group? Answer: The hydroxyl group is the strongest activating group, and the position ortho to the methoxyl is too sterically hindered. 21 Experiment 33: Aromatic Iodination 22