Defects in Crystalline Solids: Lecture Notes
advertisement

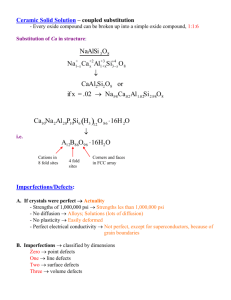
Chapter 5 Highlights: 1. Be able to recognize and discriminate between different point and extended defects in metals and ceramics. Know the direction of the Burger's vector for extended defects. 2. Given the necessary information, be able to determine the vacancy concentration, the vacancy fraction, or the activation energy for vacancy formation in a crystalline solid. 3. Understand the factors that affect metal solubility and be able to give rough predictions of when metals are mutually soluble. Notes: 1. 2. Perfect infinite lattice of chapter 3 does not exist, with the exception of silicon for the microelectronics industry. There are two reasons for this: Thermodynamics: Maximizing entropy (disorder) causes the introduction of defects and impurities. In other words, even at thermodynamic equilibrium, defects still exist. Kinetics: If a material is quenched from high to low temperature, non-equilibrium structures can be frozen in. In other words, the defect is not the lowest energy (or highest entropy) state, but the kinetics for defect removal are so slow that for all practical purposes, the defect will remain forever. Materials properties often depend strongly on defects. Very small defect densities can cause yield losses in the semiconductor industry. Defects can now be imaged by scanning probe microscopy: Defects Start with point defects: A) Vacancy: Show figure 5.1 (above). - Q /kT Nv = N e v ln N v = ln N - log N v = log N - Qv kT Qv 2.303kT Here N is the # of atomic sites per unit volume, N v is the number of vacancies per unit volume, Q v is the activation energy for vacancy formation, k is Boltzmann's constant, and T is the absolute temperature (Kelvin or Rankine). A graph of ln N v (or log N v ) versus 1/T is called an Arrhenius plot and should yield a straight line. The slope of this line is equal to-Q v/k (or -Q v /2.303k). You should absolutely be able to draw an Arrhenius plot before you finish this course. This is important because many materials properties show an exponential dependence on temperature. Example: Given that the density and atomic weight of Fe are 7.65 g/cm3 and 55.85 g/mol, respectively, and that the activation energy for vacancy formation is 1.08 eV/atom, what is the concentration of vacancies at 850°C? In equation (5.1), the only unknown is N, the number of sites per unit Volume. We can calculate that by dimensional analysis or by using equation (5.2): N = N = ρ NA A (7.65 g / cm )(6.023 x10 atoms / mol ) (55.85 g / mol )) 3 23 N = 8.25 x10 22 atomic sites / cm 3 Now use equation (5.1) to calculate the vacancy concentration: 1.08 eV 22 3 N v = (8.25 x10 / cm ) exp − −5 (8.62 x10 eV / ° K )(850 + 273° K ) N v = 1.18 x10 18 / cm 3 Back to Listing of Defects B) Self-interestitial (very rare): Show figure 5.1 again. Now consider defects in ceramics, which are somewhat different from those in metals. In a ceramic, electrical neutrality must be maintained. Therefore if a cation is missing, then this charge must be balanced either with a missing anion, or with an extra cation somewhere else. For this reason, point defects in ceramics tend to occur as pairs of sites. C) Frenkel defect: This involves a coupled cation vacancy and a cation interstitial. Show figures 5.2 and 5.3. Why is there no defect consisting of a coupled anion vacancy and anion interstitial? Remember, anions tend to be much bigger than cations, so anion interstitials are quite rare. D) Schottky defect: This involves a coupled cation vacancy and anion vacancy. Impurities in Solids Most industrial metals are alloys containing more than one metal. By combining 2 metals one can vary the properties of the material over a wide range and achieve more design flexibility. Sterling silver is 92.5% Ag and 7.5% Cu. Silver is very corrosion resistant but is quite soft. Ag/Cu is still quite corrosion resistant but is much harder. When 2 metals are mixed, you obtain either 1) Solid solution- 2 metals distributed randomly within one crystal structure. The best known 2) 3) example is Cu and Ni. Phase separation- 2 metals may separate like oil and water. Metallic example: Pb and Sn. Intermetallic compound- 2 metals have directional bonds as in covalent solids such as SiO2 . Metallic example: Mg2 Pb. Solvent: Solute: Majority species Minority species Solubility can be predicted from the Hume-Rothery rules (guidelines): 1) 2) 3) 4) Atomic radius, difference must be <15% to get solubility. Later we will see a phase diagram of Sn (R=1.40A) and Pb(R=1.75A), which have very limited solubility at room temperature (see figure 9.7). Electronegativity should be similar, otherwise they will form an intermetallic compound. The electronegativities of Mg and Pb are 1.2 and 1.8, respectively, so they form a compound Mg2 Pb (see figure 10.20). The crystal structure must be the same or the metals will phase separate. Cu is FCC, Zn is HCP, see figure 10.19. The two species must have similar valence. These should be viewed as general guidelines rather than rigid rules. The best way to determine solid solubility is from the phase diagram for a particular pair of elements. This will be introduced in chapter 10. Perfect solid solution- Cu and Ni For any composition from 100% Cu to 100% Ni, these two materials form a FCC crystal strucuture with each of the two components distributed randomly. Perform checks above: R Cu = 0.128 nm, R Ni = 0.125 nm, 2.4% difference. Electronegativities are 1.9 and 1.8. Both have a FCC crystal structure. Valences are ±1, ±2. Although this discussion has centered on metals, 2 different ceramics can also form solid solutions. An example is Al 2 O3 and Cr 2 O3 , which are completely miscible, just like Cu and Ni. The rest of the defects that will be mentioned can occur in both metallic and ceramic crystalline solids. Back to Listing of Defects E) Substitutional Impurity. Show figure 5.5. F) Interstitial Impurity: This occurs for atoms with very small R, such as C(.071 nm) in Fe(R=.124 nm). C in Fe produces steel and will be covered later in the semester. Obviously steel is an important industrial material, and this will be covered extensively in chapters 10 and 11. Show figure 5.5 again. Specification of Composition For alloys of two or more metals (or semiconductors or ceramics), the composition is usually given in weight %, but may also be given in atom %. Although weight % is easier to measure and more convenient, atom % provides a more accurate measure of stoichiometry. For example, later in this course, intermetallic compounds such as Mg 2 Pb will only be recognized within phase diagrams when one knows the atom % of each element. The composition in terms of weight % is given as: C1 = m1 x100 m1 + m 2 The composition in terms of atom % is given as: C1 ' = n1 x100 n1 + n2 The textbook provides some conversions: C1 ' = C 1 A2 x100 C 1 A2 + C 2 A1 C2 ' = C 2 A1 x100 C 1 A2 + C 2 A1 C1 = C 1 ' A1 x100 C 1 ' A1 + C 2 ' A2 C2 = C 2 ' A2 x100 C 1 ' A1 + C 2 ' A2 For some diffusion calculations, we will want to know the concentration in terms of mass per unit volume, C i ”. We have the further equations: C1 " = C1 C1 ρ1 C2" = + C2 ρ2 C2 C1 ρ1 + x1000 C2 x1000 ρ2 Here the concentration is given in kg/m3 when the densities are given in g/cm3. You must follow these units for these equations to be correct. The textbook also provides formulae for calculating the average density and average atomic weight of a binary alloy. Example: Mo forms a substitutional solid solution with W. Compute the # of Mo atoms per cm3 for a Mo-W alloy containing 16.4 wt % Mo and 83.6 wt % W. Their densities are ρ Mo = 10.22 g/cm3 and ρ W = 19.30 g/cm3. First use equations (5.12) to convert from wt % to mass per unit volume: C Mo " = C Mo x1000 C Mo CW + ρ Mo C Mo " = ρW 16.4 x1000 16.4 83.6 + 10.22 19.3 C Mo " = 2763 kg / m 3 Now we have to convert these units into atoms/cm3: (2763 kg / m )(1000 g / kg )(6.023 x10 "= 3 C Mo 23 atoms / mole (95.94 g / mole )(100 cm / m )3 ) C Mo " = 1.73 x10 22 atoms / cm 3 Back to Listing of Defects Now move from point defects to extended defects, which include dislocations. G) Edge dislocation- show figure 5.8. Dislocation line is formed by an extra half-plane of atoms. Burger's vector b is perpendicular to the dislocation line. The Burger’s vector is the displacement vector necessary to close a stepwise loop around the defect. Show figures from Shackleford. H) Screw dislocation- show figure 5.9. Upper front of crystal is shifted one atomic position. Burger's vector b is parallel to the dislocation line. I) Mixed dislocation- show figure 5.10. More common than edge or screw, has 2 dislocation lines. But b vector remains constant along entire extent of dislocation. J) Grain boundaries- show figure 5.11. K) Twin boundary- show figure 5.13. Mirror symmetry across plane. Microscopic Examination: Optical microscope- Resolution limited to the light wavelength, which varies from 390-750 nm. Electron microscope- Resolution limited to the electron wavelength. Remember that electron’s have a de Broglie wavelength of: λ = h = p h 2mE Here λ, h, p, m and E are the wavelength, Planck’s constant, momentum, electron mass, and energy. Here E is given in J. If we substitute for the electron mass, electron charge, Planck’s constant, and remembering that the energy in eV is equal to the voltage through which the electron is accelerated divided by the charge on an electron, then h λ = = p h 150.4 = 2mqV V 1/ 2 Here E is given instead in eV and λ. Thus, a simple relationship exists between the electron acceleration voltage and the electron wavelength. This allows one to tune the wavelength by tuning the acceleration voltage and thus probe a wide variety of length scales. For example, λ = 10 nm for150.4 V electrons, but λ = 1 nm for 15,040 V electrons. The scanning electron microscope (SEM) (we have two on the 3rd floor of the CAMP building) obtains images by directing an electron beam at a sample, collecting the secondary electrons, and rastering the electron beam across the sample surface. The TEM (3rd floor of the CAMP building) collects images of the transmitted electrons that pass through ultrathin samples. We have created multimedia software for a virtual SEM that can be used to learn how to use this instrument. The software is available at: www.clarkson.edu/~thinfilm and www.clarkson.edu/~thinfilm/sem/intro.html. High-resolution atomic images can be obtained using scanning tunneling microscopy (STM) and atomic force microscopy (AFM). During STM, a voltage is applied to a sharp metal tip that is scanned across the sample surface, with the tip-sample distance continuously adjusted to maintain a constant current (nA range). This directly records the surface topography. For materials with limited conductivity, the AFM can be used to image surface topography using displacement of a sensitive cantilever. Clarkson has AFMs in several professors’ laboratories. Grain Size Determination: One needs to quantify the grain size that is observed in electron micrograph images. One method defines this according to the grain size number, as defined by the American Society for Testing and Materials (ASTM): N = 2 n −1 n is the grain size number. N is the average number of grains per square inch at a magnification of 100x. Example: Determine the ASTM grain size number of a metal specimen if 45 grains per square inch are measured at a magnification of 100x. For the same specimen, how many grains per square inch will there be at a magnification of 85x? Start with Equation (5.19): N = 2 n −1 Taking log of both sies: log N = ( n − 1) log 2 Solving for n: n= log N +1 log 2 Substituting: n= log 45 + 1 = 6.5 log 2 For magnifications other than 100x, a modified version of Equation (5.19) can be used: 2 M n −1 NM =2 100 Solving for NM NM = 2 n −1 100 M 2 Substituting: 2 NM = 2 6.5−1 100 2 = 62.6 grains / in 85