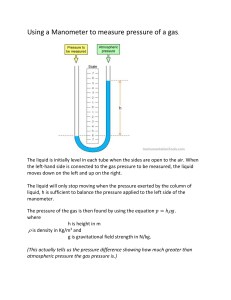
AP Chemistry Manometer Practice Problems
advertisement

AP Chemistry Manometer Practice Problems 1. A geochemist heats a limestone sample and collects the CO2 released in an evacuated flask attached to an open-ended manometer. If the barometer reads 754 mmHg and the difference in mercury heights in the manometer is 6.8 inches (with the low side on the atmospheric pressure side), calculate the pressure from the CO2 in both mmHg and psi. Draw a picture of this manometer as well. 2. That same geochemist in (1), wary about the long term effects of mercury, decides to make a manometer out of glass tubing and water. If the results of the experiment are identical to that of (1), what would the pressure of the CO2 gas be, in mmHg?