S P D F Orbitals
advertisement
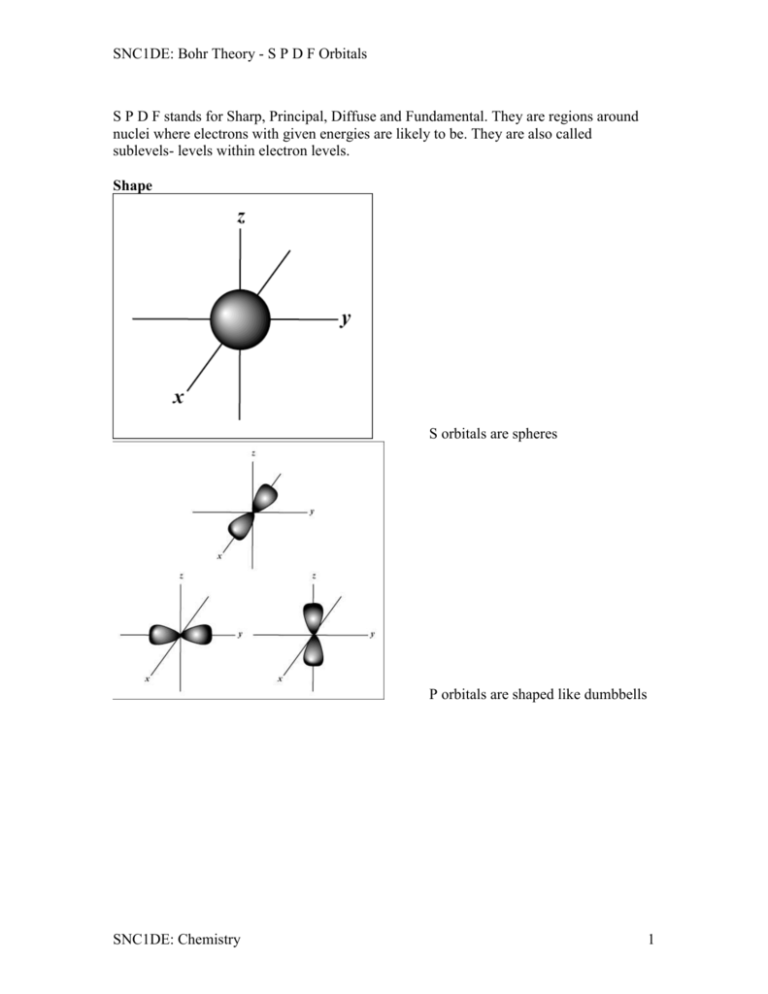
SNC1DE: Bohr Theory - S P D F Orbitals S P D F stands for Sharp, Principal, Diffuse and Fundamental. They are regions around nuclei where electrons with given energies are likely to be. They are also called sublevels- levels within electron levels. Shape S orbitals are spheres P orbitals are shaped like dumbbells SNC1DE: Chemistry 1 SNC1DE: Bohr Theory - S P D F Orbitals D orbitals F orbitals What kind of elements? Groups 1 & 2 on the periodic table are S elements Groups 13-18 on the periodic table are P elements Groups 3-12 on the periodic table are D elements Inner Transition Metals on the periodic table are F elements How many electrons can be in each one? S sublevels can have 2 electrons P sublevels can have 6 electrons D sublevels can have 10 electrons F sublevels can have 14 electrons Differences between them Their energy Their levels of electrons The types of elements they shown up in. SNC1DE: Chemistry 2 SNC1DE: Bohr Theory - S P D F Orbitals Electron Configurations and the Periodic Table The periodic table is your best guide to the order in which orbitals are filled. 1s 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s 5d 6p 7s 6d 4f 5f The electrons fill up in the following order: 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p 5d 5f Orbitals of a subshell will fill with electrons with the same spin first then with electrons of opposite spin. Electron Configurations The way in which the electrons are distributed among the various orbitals of an atom are called its electron configuration. SNC1DE: Chemistry 3 SNC1DE: Bohr Theory - S P D F Orbitals The most stable, or ground state , electron configuration of an atom is that in which the electrons are in the lowest possible energy states. paired unpaired Li 1s 2s ms=+1/2 ms= -1/2 1s 2s 2p 3s Li Be B C N Ne Na The filling of the 2p subshell is complete at neon, which has a stable configuration with eight electrons (an octet) in the outermost shell. SNC1DE: Chemistry 4






