Osmosis and Gummy Bears Purpose: To investigate water
advertisement

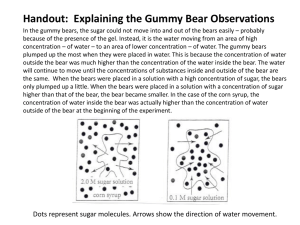
Osmosis and Gummy Bears Purpose: To investigate water movement into and out of a highly concentrated body depending on the concentration of the substance surrounding it. Background Info: Osmosis is the movement of water from an area of high concentration to an area of low concentration. Isotonic solutions are solutions in which the concentration inside a body is the same as on the outside. Hypertonic solutions are solutions in which the concentration inside a body is lower than the concentration outside a body. Hypotontic solutions are solutions in which the concentration inside a body is higher than the concentration outside a body. Hypotheses: 1. If someone soaks Gummy Bears in water, then the size of the bears will (increase, decrease, remain the same. Circle your answer.) because _________________________________________________________ ______________________________________________________________________________________ 2. If someone soaks Gummy Bears in a sugar and water solution, then the size of the bears will (increase, decrease, remain the same. Circle your answer.) because _________________________________________ ______________________________________________________________________________________ Materials: 2 beakers permanent marker 2 wire mesh squares 2 Gummy Bears (different colors) saturated salt solution (6 oz per cup) tap water centimeter ruler Masking tape scale Procedure 1. Obtain two beakers, two different colored Gummy Bears, and a ruler. 2. Using a permanent marker and masking tape, label one beaker “water” and one “sugar water”. Also include your names and class period. 3. Measure your bear (in cm) from top to bottom (length) and from side to side (width) and from front to back (height). Record the centimeters in the data table. Use decimals. 4. Fill both beakers with 75 mL of water. 5. Weigh out 12 g of sugar. Put the sugar in the beaker labeled “sugar water” and stir until the sugar is dissolved. CAREFUL WITH THE GLASS!!! 6. Place the cups on the side counter in the space marked for your period. Let them sit overnight. 7. After 24 hours, gently pour the water out of each beaker over a screen into a sink. BE CAREFUL not to break the bears; they are very fragile. Catch each bear on a separate screen. 8. While the bears are on the screen, measure the length, width, and height for each bear. Record. Blot the screen dry by placing it on a paper towel. 9. Calculate the volumes of each bear (l x w x h). Size of Gummy Bears before and after being soaked in regular and in sugar water. Length Width Height Volume Regular water bear before soaking Salt water bear before soaking Bear after soaking in regular water After soaking in salt water DONT FORGET UNITS!!! Conclusions: 1. What happened to the bears when placed in distilled water? Why? 2. What happened to the bears when placed in tap water? Why? 3. What happened to the bears when placed in salt water? Why? 4. What do you think would have happened to the bears if, after the last day, they were again placed in distilled water? 5. Calculate the percent change in volume after each step of the experiment. % change in volume = (final volume - initial volume)/ initial volume x 100 6. Place the percentages in the table below: Percent change in volume of Gummy Bears after being soaked in either regular water or sugar water % Change in Volume Regular Water Bear Sugar Water Bear 7. Make a bar graph of the percent changes. Label axes. Place a scale on the vertical axis for percent change and give a title for the graph. Place the data for both bears on the same graph. If you have a negative value for a percent change, start the vertical axis at a negative number. (For example: -50, -25, 0, 25, 50, 75, 100, etc.) 8. Write a paragraph on a separate piece of paper which explains the results of this experiment using the concept of osmosis. Make sure to use the words isotonic, hypertonic, hypotonic, equilibrium, concentration gradient, and osmosis. Include your data where appropriate to explain your results and conclusions. Include any further questions you might have about this experiment and any sources of error. This will be worth at least half of the lab so make it detailed and well thought-out.