Observing Osmosis Using Gummy Bears
advertisement

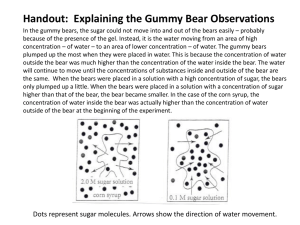
Observing Osmosis Using Gummy Bears Background Information: Movement through membranes is called transport. Molecules are in constant motion, and tend to move from areas of higher concentrations to lesser concentrations. Diffusion and osmosis are passive forms of transport; this means that they do not need energy to move areas of high concentration to areas of low concentration. Active transport requires energy to transport molecules from low concentration to high concentration. Osmosis is the movement of water through a selectively permeable membrane from an area of high concentration to an area of low concentration. Gummy Bears are popular candies made of gelatin, starch, and sugar. Question/Problem: How will soaking Gummy Bear candies in distilled water affect the size of the candy? Prediction/Hypothesis: (explain your prediction based on the background information – write in your lab NOTEBOOK). Materials: Beaker, Water, Gummy bear, Ruler, Electronic Balance, Calculator Length Width Height Procedure: Day 1 1. Obtain a 50mL beaker, a gummy bear and a ruler. 2. Label the cup with your group's name and class period 3. Measure your bear (in cm) from top to bottom (length), from side to side (width), and from front to back (height). Record these measurements in your data table. 4. Place the bear in the beaker and fill with 40mL of water. Place the beaker in the designated area for 24 hours. 5. Calculate the volume of each bear (l x w x h). Day 2 7. Use a plastic spoon to gently scoop out each bear. BE CAREFUL not to break the bears, they are very fragile. 8. 9. 10. 11. 12. 13. Measure the length, width, and height and record in your data table. Make any observations about the color of the bears and/or the water. Empty the water out of the beaker. Fill the cup with 40mL of salt water solution. Return the bears to the beaker and return them to the designated area for another 24 hours. Calculate the volume of each bear for Day 2. Day 3 Use the spoon to gently scoop out each bear and take its measurements. 15. Make any observations about the color of the bears and/or the water. 16. Rinse out the beaker and throw the gummy bear away. 17. Calculate the volume of each bear on Day 3. 14. Data: (create the following table in your lab notebook – use a RULER!!) Before Soaking in water After soaking in water After soaking in salt water (Day 1) (Day 2) (Day 3) Height (H): Height (H): Height (H): Width (W): Width (W): Width (W): Length (L): Length (L): Length (L): Volume (LxWxH): Volume (LxWxH): Volume (LxWxH): Mass (in grams): Mass (in grams): Mass (in grams): Descriptive observations: Descriptive observations: Descriptive observations: Calculate the percent change in the size of the candy – write out AND record in your notebook!! % CHANGE IN VOLUME = (After soaking volume – Before soaking volume / Before soaking volume) x 100 ( _________ - _________ / ________ ) x 100 = _____% % CHANGE IN MASS = (After soaking mass – Before soaking mass / Before soaking mass) x 100 ( _________ - _________ / ________ ) x 100 = _____% Graph: Create a graph that shows time vs. volume of Gummy Bear. You will show the change in solution by changing the color of the line on the graph. Remember to place your independent variable on the x-axis of your graph and the dependent variable on the y-axis. Conclusions: Day 1-2 Questions: 1. What is the purpose of measuring the bear's volume? 2. What happened to the bears after being initially placed in water? (Look at your Day 1 vs. Day 2 results.) Explain the direction of water movement. 3. Draw a diagram of what happened to the gummy bear over the first 24 hours. 4. Explain what type of solution you are working with. (Hypotonic, hypertonic, Isotonic) 5. What would eventually happen if it was left in the water for a few more days? Day 2-3 Questions: 6. What happened to the bear that was placed in the salt water? Explain the direction of water movement. 7. Draw a diagram of what happened to the gummy bear over the second 24 hours. 8. Explain what type of solution you are working with. (Hypotonic, hypertonic, Isotonic) 9. Explain any change in color in the bears or their solutions. Describe the movement of water that produced the results. 10. What does it have to do with diffusion and the cell membrane? Conclusion: Write a short paragraph to explain the results of this investigation using the concept of osmosis. Include specific data to support what you say.