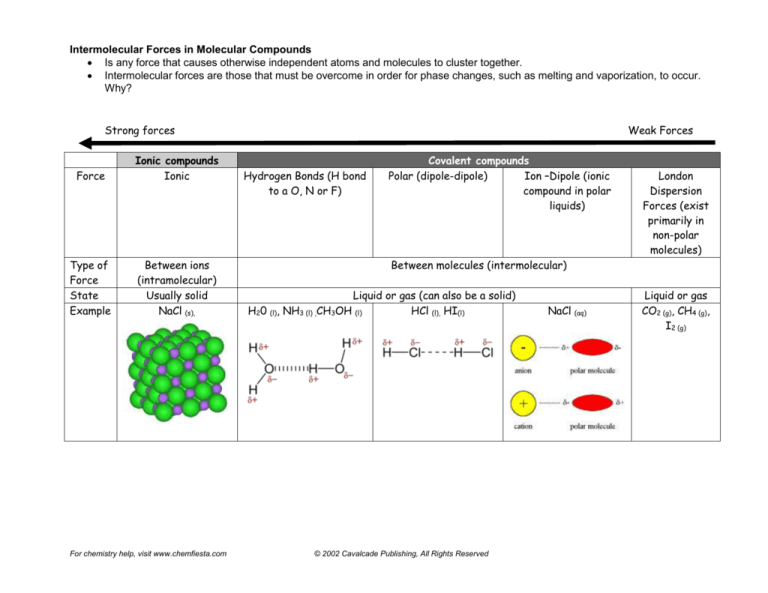
Intermolecular Forces in Molecular Compounds
Is any force that causes otherwise independent atoms and molecules to cluster together.
Intermolecular forces are those that must be overcome in order for phase changes, such as melting and vaporization, to occur.
Why?
Strong forces
Force
Ionic compounds
Ionic
Type of
Force
State
Example
Between ions
(intramolecular)
Usually solid
NaCl (s),
For chemistry help, visit www.chemfiesta.com
Weak Forces
Hydrogen Bonds (H bond
to a O, N or F)
Covalent compounds
Polar (dipole-dipole)
Ion –Dipole (ionic
compound in polar
liquids)
London
Dispersion
Forces (exist
primarily in
non-polar
molecules)
Between molecules (intermolecular)
Liquid or gas (can also be a solid)
H20 (l), NH3 (l) ,CH3OH (l)
HCl (l), HI(l)
© 2002 Cavalcade Publishing, All Rights Reserved
NaCl (aq)
Liquid or gas
CO2 (g), CH4 (g),
I2 (g)
For chemistry help, visit www.chemfiesta.com
© 2002 Cavalcade Publishing, All Rights Reserved
Intermolecular Forces and Properties of Compounds
1. For the molecules in question 1, indicate the type(s) of intermolecular forces
present.
a. H2O _____________________________
b. NH3 ______________________________
c. CF4 ______________________________
d. I2 ________________________________
e. NCl3 ________________________________
2. In each of the following problems, rank the molecules from lowest to highest polarity. Consider the polarity
of the individual bonds as well as molecular polarity
a) PF3, LiOH, SF2
b) Ni(OH)3, HCl, CH3OH
3. Name the strongest intermolecular force present for each of the following compounds:
ammonia
___________________________________
boron trichloride
___________________________________
water
___________________________________
carbon tetrachloride
___________________________________
ethane (C2H6)
___________________________________
methanol (CH3OH)
___________________________________
borane (BH3)
___________________________________
5. Consider the following substances: HF(aq) SO2(g)
PCl3 F2 (g)
FeCl2
a) Which of the following substances is most likely to exist as a crystalline solid at room temperature?
Why?
b) Of the gases, which would be the hardest to condense to a liquid under pressure? Why?
c) Which of the following is expected to have the highest boiling point?
d) Which is expected to have the lowest?
For chemistry help, visit www.chemfiesta.com
© 2002 Cavalcade Publishing, All Rights Reserved
Ranking Molecules by Increasing Polarity
Solutions
In each of the following problems, rank the molecules from lowest to highest polarity:
1)
PF3, LiOH, SF2, NF3
NF3 < PF3 < SF2 < LiOH
2)
Ni(OH)3, N2H2, CH3OH, C2H5OH
N2H2 < C2H5OH < CH3OH < Ni(OH)3
3)
B2F4, H2C2O4, CuCl2, CF2O
B2F4 < H2C2O4 < CF2O < CuCl2
4)
PH3, PF3, NH3, NF3
PH3 < NH3 < NF3 < PH3
5)
H2O, H2S, HF, H2
H2 < H2S < H2O < HF
4. Which of the following substances is most likely to exist as a crystalline solid at room temperature? Of the
gases, which would be the hardest to condense to a liquid under pressure?
a) HF d) SO2
b) PCl3 e) F2
c) FeCl2
5
Which of the following is expected to have the highest boiling point?
Which is expected to have the lowest?
a) CO2 b) Ar c) CF4
d) LiCl e) SiF4
3. For each of the pairs below, circle the substance with the highest boiling point:
a. gasoline or salad oil
b. O2 or N2
c. NH3 or NCl3
d. CH3CH2CH3 (propane) or CH3CH2CH2CH3 (butane)
4. Define electronegativity. How does it relate to the intermolecular forces between
For chemistry help, visit www.chemfiesta.com
© 2002 Cavalcade Publishing, All Rights Reserved
molecules?
5. Define
For chemistry help, visit www.chemfiesta.com
© 2002 Cavalcade Publishing, All Rights Reserved







