Chapter 6 Review
advertisement

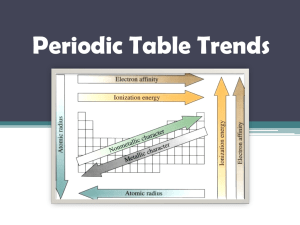
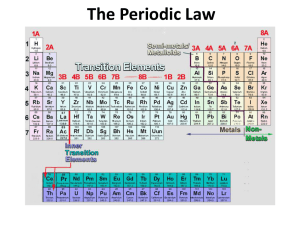
Chapter 6 Review 1. The elements of the periodic table are sorted into groups according to what? 2. Who was the scientist that first arranged the elements into a periodic table? 3. He arranged the elements according to their what? 4. In the modern periodic table the elements are arranged according to their ____________. 5. When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties is known as the _______________________________. 6. Vertical columns in the table are known as _____________________. 7. Horizontal rows are called ________________________. 8. The class of elements that are ductile and malleable are the ___________. 9. Group IA elements are known as the ____________________. 10. The group of elements that have their highest occupied energy level filled are the ___________________. 11. The elements whose outermost s sublevel and the d sublevel contain electrons. 12. The elements where the f sublevel contains electrons. 13. The name of the group of elements whose electron configuration is s2p5 15. Outermost s and p electrons or electrons that occupy the highest energy level. 16. Electrons not in the outermost energy level, the inner electrons. 17. The 1A- 7A elements are known as the ________________________ elements. 18. Write the short-hand electron configuration for Fluorine. 19. What element ends with an electron configuration of [Ar]4s23d7. 20. Write the short hand electron configuration for lead. 21. The energy required to remove an electron from a neutral, gaseous atom is known as the _____________. 22. The trends in atomic radius __________________ across and ___________________ down the table. 23. What factor is responsible for the trend in atomic radius down the group? 24. What factor is responsible for the trend in atomic radius across the period? 25. A postiviely charged ion. 26. A negatively charged ion. 27. Which is the smallest radius? P, S, As, or Se? 28. Which of the atoms in 27 is the largest atom? 29. Put the atoms in 27 in order of increasing radius. 30. The trends in ionization energy _________________ across a period and _____________ down the groups. 31. Which has the largest ionization energy? Ca, Sr, Ba, Mg? 32. What factors effect the ionization energy across a period? 33. What factor effects the ionization energy down a group? 34. B, C, N, O - which has the highest ionization energy? 35. Anions are ____________than their parent atoms, while cations are ________________. ANSWERS: 1. properties 2. Mendeleev 3. atomic mass 4. atomic number 5. periodic law 6. groups 7. periods 8. metals 9. alkali metals 10. noble gases 11. transition metals 12. inner transition metals 13. halogens 14. 15. valence electrons 16. core electrons 17. representative 18. [He]2s22p5 19. Co 20. [Xe]6s24f145d106p2 21. ionization energy 22. decreases, increases 23. an additional FILLED energy level 24. the increasing effective nuclear charge 25. cation 26. anion 27. S 28. As 29. S, P, Se, As 30. increase, decrease 31. Mg 32. increasing effective nuclear charge, decreasing atomic radius 33. increasing atomic radius 34. N (it's one of the exceptions) 35. larger, smaller