Soybean peroxidase (SbP, RZ 2.8) was a gift from Bio
advertisement

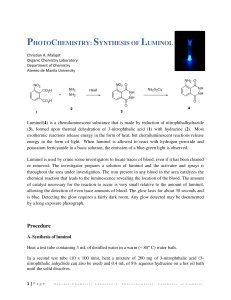
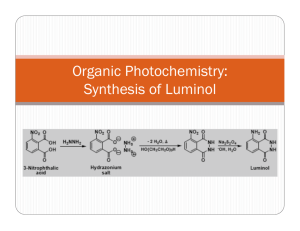
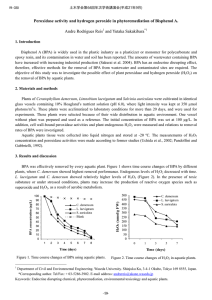
Experimental Section Soybean peroxidase (SbP, RZ 2.8) was a gift from Bio-Researching Products Ldt. (USA) and used without further purification. Sodium 3-(10’-phenothiazinyl)propane-1-sulfonate was synthesized as described in [1]. 4-Morpholinopyridine, 4-dimethylaminopyridine and 4pyrrolidinopyridine were from Aldrich (USA); luminol, hemine and Tris were from Sigma (USA), H2O2 (30%) from ChimMed (Russia). Pyridine and dimethylaminobenzene were a gift of Dr. A. Khomutov (Engelhardt Institute of Molecular Biology RAS, Russia). The H2O2 concentration was determined by monitoring A240, using = 43.6 M-1cm-1 [2]. The required dilutions of H2O2 were prepared daily. Black polystyrene plates (high protein binding) were obtained from Nunc (Demark). The enhanced CL reaction was carried out as follows [3]: 245 μL of 50 mM Tris-HCl, pH 8.3 containing 0.5 mM hydrogen peroxide, 0.75 mM luminol, 0.75 mM SPTZ and 1 mM secondary enhancer were placed in wells of black polystyrene plates for chemiluminescent enzyme immunoassay. Then the enzymatic reaction was initiated by adding 5 μL of 350 pM SbP soluble in the same buffer. CL intensity was measured at room temperature on a luminometer Zenyth 1100&3100 (Anthos, Austria). The light intensity was expressed in relative chemiluminescence units (RLU). Kinetics of change of SPTZ●+ concentration upon SbP-catalyzed oxidation of SPTZ in the absence and presence of MORPH were carried out by monitoring absorbance at 513 nm [4] on spectrophotometer UV-2401 (Shimadzu, Japan). The experiment was performed as follows: 3.7 μL SbP (2.7×10-6 M) or 6.3 μL hemine (1.6×10-3 M) were added to 1 mL of 50 mM Tris, pH 8.3, containing SPTZ (1.0 mM), H2O2 (0.5 mM ) and MORPH (0 or 1 mM). The values of the initial rate of SPTZ●+ production in the absence and presence of MORPH were estimated as the slope of the kinetic curves at time tending to zero. The experimental data of the decomposition of SPTZ●+ obtained in the absence and presence of MORPH (flowing branches of the kinetic curves) were analyzed using an equation of second-order reaction. [1] E. Marzocchi, S. Grilli, L. della Ciana, L. Prodi, M. Mirasoli, A. Roda, Chemiluminescent detection systems of horseradish peroxidase employing nucleophilic acylation catalysts, Analyt. Biochem. 377 (2008) 189–194. [2] R. J. Kulmacz, Prostaglandin H synthase and hydroperoxides: peroxidase reaction and inactivation kinetics, Arch. Biochem. Biophys. 249 (1986) 273–285. [3] M. M. Vdovenko, L. Della Ciana, I. Yu. Sakharov, 3-(10'-Phenothiazinyl)propane-1sulfonate is a potent enhancer of soybean peroxidase-induced chemiluminescence, Analyt. Biochem. 392 (2009) 54–58. [4] M. M. Vdovenko, A. Ch. Vorobiev, I. Yu. Sakharov, Phenothiazines devivatives as enhancers of peroxidase-dependent chemiluminescence, Bioorg. Chem. (2012), in press