Ex 8 Luminol.pptx
advertisement

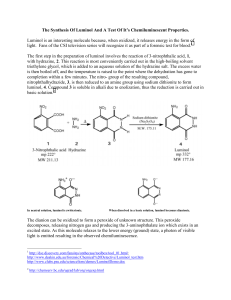
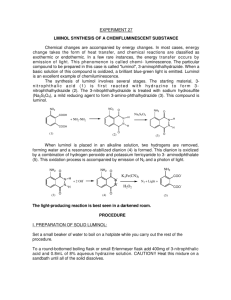
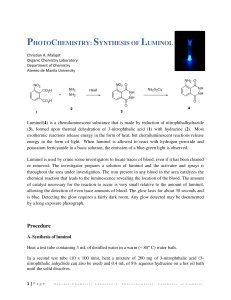
Organic Photochemistry: Synthesis of Luminol Introduction Purpose: Demonstrate how a chemical process can lead to a photochemical outcome through the synthesis of a chemiluminescent substance, luminol. Other examples of chemiluminescent compounds: luciferacse (produces light in fireflies) Cyalume® (used in glow sticks) Photochemical Processes Light production is related to the electronic state of the molecule (typically electrons are paired). If an electron is promoted to an orbital of higher energy, it is no longer paired (therefore no longer constrained by the Pauli principle). If two unpaired electrons have: parallel spins = triplet state opposite spins = singlet state Photochemical Processes Transitions where spin changes (ground state with opposite spins to triplet state with parallel spins) are “forbidden”. This spin change (ground state to triplet state) violates the postulate that angular momentum most be conserved. However, they do happen through indirect means and tend to be highly reactive. Luminol is a precursor for an intermediate in the triplet state that will produce light through fluorescence. Synthesis of Luminol • High boiling solvent, such as triethylene glycol (bp 290 ˚C), allows for the dehydration of hydrazonium salt to afford 3nitrophthalhydrazide. • The nitro-compound is reduced with sodium hydrosulfite forming luminol. Light Producing Reaction Energetics of the Chemiluminescent Procedure Begin to heat 10-15 mL of water in a small beaker for later use (near boiling). Into a 25mL RBF, place 0.300 g of 3-nitrophthalic acid and 600 µL of 8% hydrazine (aq.). Add a boiling chip and heat the mixture gently to dissolve the solid (may not totally dissolve). Add 1.0 mL of triethylene glycol. Boil off the water, then allow the temperature to rise to 215 ˚C. Keep at this temp for 3-5 minutes. Allow the solution to cool to 100 ˚C then add 4.5 mL of the previous prepared hot water. Cool the flask in running water, and place in ice to help form a precipitate. Collect the yellow solid by vacuum filtration ( ≈210 mg.) Procedure Place the product (moist is O.K.) into an Erlenmeyer along with 1.5 mL of 3M NaOH and 0.90 g of sodium hydrosulfite (should be brown-red in color). Heat the mixture to boiling, stir, and maintain boiling for 5 minutes (occasionally stir with stirring rod). Cool the flask with running water. Carefully add 0.60 mL of acetic acid and make sure the solution is acidic with pH paper. Collect precipitate (luminol) by vacuum filtration. Allow product to dry on funnel as well as clay plate for 5 minutes. In a 50 mL flask (labeled “Solution A”), mix 60 mg of luminol with 2 mL of 3M NaOH and dilute with 18 mL of water. In a second 125 mL flask (labeled “Solution B”), mix 4 mL of 3% aqueous Potassium ferricyanide, 4 mL of 3% hydrogen peroxide and 32 mL of water. In a dark room, add the components of solution A to solution B. Note what happens. Grading Rubric Pre-lab Procedure % Yield Final Product Glows!!!!!!