Unit 8 - Mole Test Review AK
advertisement
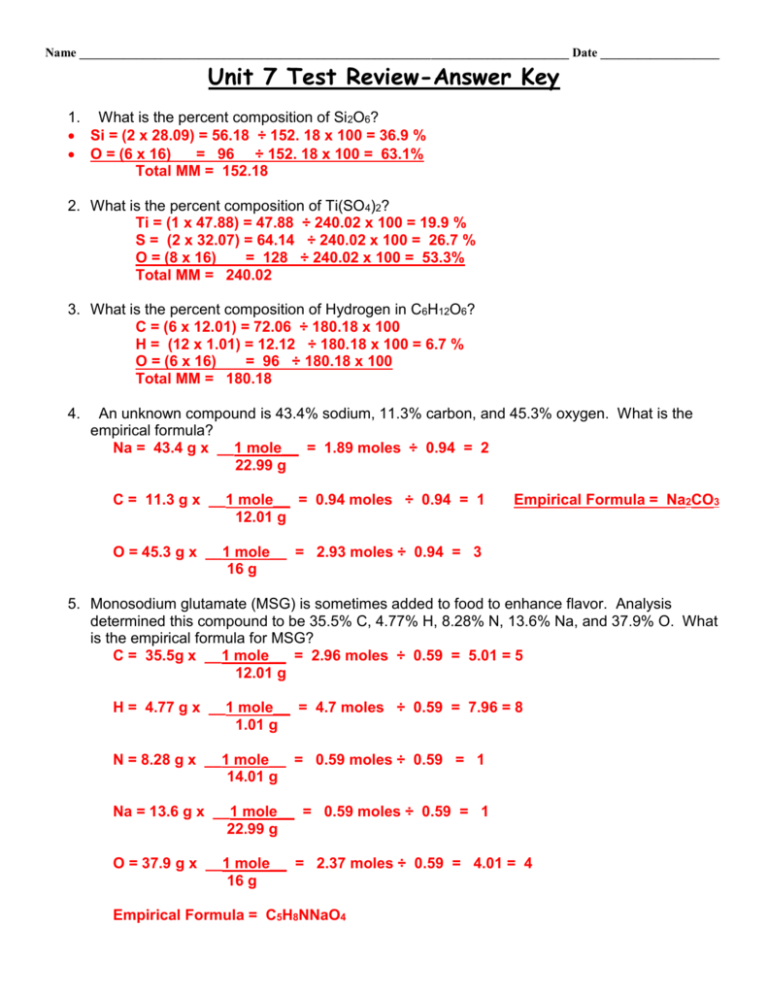
Name ______________________________________________________________________________ Date ___________________ Unit 7 Test Review-Answer Key 1. What is the percent composition of Si2O6? Si = (2 x 28.09) = 56.18 ÷ 152. 18 x 100 = 36.9 % O = (6 x 16) = 96 ÷ 152. 18 x 100 = 63.1% Total MM = 152.18 2. What is the percent composition of Ti(SO4)2? Ti = (1 x 47.88) = 47.88 ÷ 240.02 x 100 = 19.9 % S = (2 x 32.07) = 64.14 ÷ 240.02 x 100 = 26.7 % O = (8 x 16) = 128 ÷ 240.02 x 100 = 53.3% Total MM = 240.02 3. What is the percent composition of Hydrogen in C6H12O6? C = (6 x 12.01) = 72.06 ÷ 180.18 x 100 H = (12 x 1.01) = 12.12 ÷ 180.18 x 100 = 6.7 % O = (6 x 16) = 96 ÷ 180.18 x 100 Total MM = 180.18 4. An unknown compound is 43.4% sodium, 11.3% carbon, and 45.3% oxygen. What is the empirical formula? Na = 43.4 g x __1 mole__ = 1.89 moles ÷ 0.94 = 2 22.99 g C = 11.3 g x __1 mole__ = 0.94 moles ÷ 0.94 = 1 12.01 g Empirical Formula = Na2CO3 O = 45.3 g x __1 mole__ = 2.93 moles ÷ 0.94 = 3 16 g 5. Monosodium glutamate (MSG) is sometimes added to food to enhance flavor. Analysis determined this compound to be 35.5% C, 4.77% H, 8.28% N, 13.6% Na, and 37.9% O. What is the empirical formula for MSG? C = 35.5g x __1 mole__ = 2.96 moles ÷ 0.59 = 5.01 = 5 12.01 g H = 4.77 g x __1 mole__ = 4.7 moles ÷ 0.59 = 7.96 = 8 1.01 g N = 8.28 g x __1 mole__ = 0.59 moles ÷ 0.59 = 1 14.01 g Na = 13.6 g x __1 mole__ = 0.59 moles ÷ 0.59 = 1 22.99 g O = 37.9 g x __1 mole__ = 2.37 moles ÷ 0.59 = 4.01 = 4 16 g Empirical Formula = C5H8NNaO4 Name ______________________________________________________________________________ Date ___________________ 6. Vanadium oxide is used as an industrial catalyst. A small sample of Vanadium oxide is determined to be 3.08 grams Vanadium and 2.42 grams oxygen. Determine the empirical formula for vanadium oxide. V = 3.08 g x __1 mole__ = 0.060 moles ÷ 0.060 = 1 x 2 = 2 50.94 g O = 2.42 g x __1 mole__ = 0.151 moles ÷ 0.060 = 2.5 x 2 = 5 16 g Empirical Formula = V2O5 7. Define empirical formula. The lowest whole number ratio of elements in a compound. 8. Which of the following is an empirical formula? a. C2H4 b. N2O c. P2O6 d. C3H6O3 9. If the molecular formula is C11H22O11, what is the empirical formula? CH2O 10. All of the following are empirical formulas EXCEPT ― a. CH4 b. N2O5 c. NH3 d. P4O10 e. H2O 11. Which one of the following is an empirical formula? a. C2F6 b. N2H4 c. H2SO4 d. P4O10 12. Which of the following is an empirical formula? a. C4H8O2 b. C2H4O2 c. C2H6O d. C6H12O6 13. Fill-in-the-blank: Molar mass of an element = The atomic mass from the Periodic table in units of g/mol . 14. What is Avogadro’s number (IN SCIENTIFIC NOTATION)? 6.02 x 1023 15. Define mole. The SI unit that measures the amount of a substance. 16. Write all the conversion factors for 1 mole. 1 moles = 6.02 x 1023 atoms, molecules, formula units 1 mole = molar mass (g) 1 mole = 22.4 L These also can/should be written as fractions. 17. Fill-in-the-blank: When setting up a conversion factor in a conversion problem, the top and bottom of the conversion factor should always be equivalent to each other. Name ________________________________________________________________________________ Date _______________ 18. How many atoms of are there in 1.25 moles Sulfur? 1.25 mol x (6.02 x 1023 atoms) = 7.53 x 1023 atoms 1 mol 19. How many moles are in 87.2 grams of SO2? SO2 molar mass = ( 1 x 32.07) + (2 x 16) = 64.07 87.2 x 1 mol = 1.36 mol 64.07g 20. If you have 3.29 x 1024 atoms of Sodium, how many liters of sodium do you have? 3.29 x 1024 atoms x 22.4 L = 122.4 L 6.02 x 1023 atoms 21. If you have 2.83 x 1022 molecules of CO2, how many moles of CO2 do you have? 2.83 x 1022 molecules x 1 mole = 0.47 moles 6.02 x 1023 molecules 22. If you have 2.12 moles of NO, how many liters of NO do you have? 2.12 moles x 22.4 L = 47.49 L 1 mole 23. If you have 1 mole of Au, how many atoms of gold do you have? (Hint: Do not calculate this question!) 1 mol = 6.02 x 1023 atoms 24. Find the molar mass of Al2(SO4)3. (26.98 x 2) + (32.07 x 3) + (16 x 12) = 342.17 g 25. If you were to calculate the molar mass of BeF2, how many moles of each element are in this compound? 1 mole of Be and 2 moles of F 26. What is the molar mass of CO2? (1 x 14.01) + (2 x 16) = 44.01 g 27. What is the molar mass of Pb3(PO4)4? (3 x 207.2) + (4 x 30.97) + (16 x 16) = 1001.48 g 28. If you have 2.2 moles of NaCl, how many grams of NaCl do you have? 2.2 moles x 58.44 g = 128.57 g 1 mole NaCl molar mass = ( 1 x 22.99) + (1 x 35.45) = 58.44 29. If you have 32.9 grams of H2O, how many liters of H2O do you have? 32.9 grams x 22.4 L = 40.9 L 18.02 g H2O molar mass = ( 2 x 1.01) + (1 x 16) = 18.02 Name ________________________________________________________________________________ Date _______________ 30. How many molecules are in 39.2 grams of Calcium? 39.2 grams x 6.02 x 1023 atoms = 5.89 x 1023 atoms 40.08 g Ca molar mass = 40.08





