SEVERAL NEW PIPERAZINEDIONE DERIVATIVES
advertisement
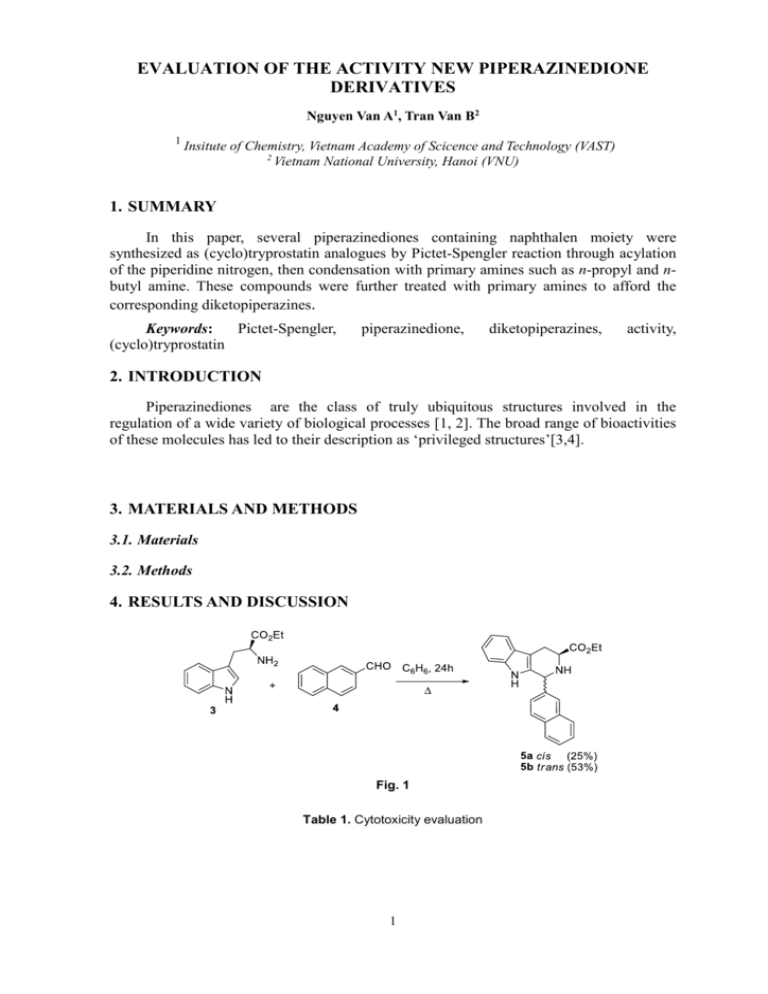
EVALUATION OF THE ACTIVITY NEW PIPERAZINEDIONE DERIVATIVES Nguyen Van A1, Tran Van B2 1 Insitute of Chemistry, Vietnam Academy of Scicence and Technology (VAST) 2 Vietnam National University, Hanoi (VNU) 1. SUMMARY In this paper, several piperazinediones containing naphthalen moiety were synthesized as (cyclo)tryprostatin analogues by Pictet-Spengler reaction through acylation of the piperidine nitrogen, then condensation with primary amines such as n-propyl and nbutyl amine. These compounds were further treated with primary amines to afford the corresponding diketopiperazines. Keywords: Pictet-Spengler, (cyclo)tryprostatin piperazinedione, diketopiperazines, activity, 2. INTRODUCTION Piperazinediones are the class of truly ubiquitous structures involved in the regulation of a wide variety of biological processes [1, 2]. The broad range of bioactivities of these molecules has led to their description as ‘privileged structures’[3,4]. 3. MATERIALS AND METHODS 3.1. Materials 3.2. Methods 4. RESULTS AND DISCUSSION Fig. 1 Table 1. Cytotoxicity evaluation 1 IC50 (µg/mL) Entry compound KB Hep-G2 LU MCF7 1 7a 62.31 >128 >128 >128 2 7b >128 >128 >128 >128 3 8a 4.40 8.00 99.2 >128 4 8b >128 >128 >128 >128 5 Ellipticine 0.625 0.625 0.625 0.625 Acknowledgements: This work was financially supported in part by scientific research and technological development project (code: ĐT.NCCB-ĐHUD.2011-G/07) 5. CONCLUSIONS 6. REFERENCES 1. Lopez-Rodr iguez, M. L.; Ayala, D.; Benham u, B.; Morcillo, M. J.; Viso, A. Curr. (2002), Med. Chem., 9: 1867– 1894. 2. Wiesner, J.; Kettler, K.; Sakowski, J.; Ortmann, R.; Katzin, A. M.; Kimura, E. A.; Silber, K.; Klebe, G.; Jomaa, H.; Schlitzer, M. Angew. (2004) Chem., Int. Ed., 43:251–254; (f) Horton, D. A.; Bourne, G. T. Smythe, M. L. (2003) Chem. Rev. 103:893–930 3. Cui, C-B.; Kayeka, H.; Osada, H. (1996) J. Antiobiot., 49: 832-835. 2