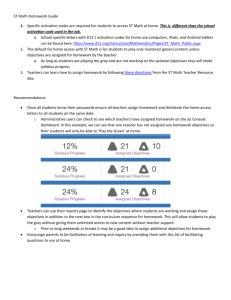
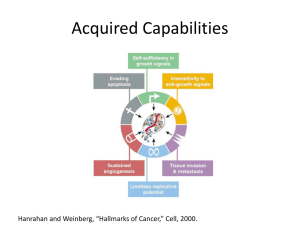
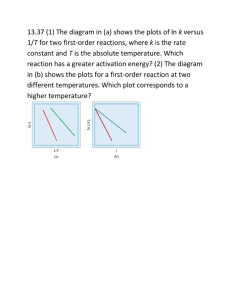
III. (Form 2) Project Description
advertisement

III. (Form 2) Project Description 1. Executive Summary (in Chinese) 面對廿一世紀後基因時代對生物科技發展及人材的培育帶來的新挑戰,我們 如何面對作出回應,會直接影響到南臺灣未來生物醫學研究及相關人材培育的成 效。我們相信解決方法必須從教育培養人才開始。成功的生物醫學教育又必須有 強大研究實力作為後盾,故此我們整合了以成大為核心,加上中山大學及國家衛 生研究院的一群優秀的研究員,其中包括國際知名的學者,或深具潛力的後進研 究員,又兼重臨床與基礎的專家,各有專長。以建立「傳承」的理念,使優秀的 研究學者不但能提昇自己,同時亦提掖後進年輕研究員,幫助他們發揮潛力。 關於研究取材方面,我們以研究細胞訊息傳遞及基因調控為主軸,推動細胞 訊息傳遞研究從傳統「線」的研究 (單一途徑研究) 進入「面」的研究階段。藉 此探討「新機制」,尋找「新基因」,進一步研究其「新功能」,以達追求研究卓 越的目標。另一方面我們亦務實地對包括膀胱癌、大腸癌、肺癌與肝癌等台灣四 種重要癌症的訊息傳遞新機制及新基因產物進行探討,利用研究細胞內訊息傳遞 途徑間的相互關係 (「面」的研究),以對本土癌症的病理機制作出貢獻,並對 影響國人健康甚巨之疾病治療有所助益。這均是後基因時代中極重要的研究題 目。 我們知道細胞受到生長因子,賀爾蒙及其他刺激物的刺激,會經由細胞內訊 息傳遞來影響某些特殊基因的表現,以達成細胞功能的發揮。然而細胞內訊息傳 遞路徑常常是多樣性的,不同的路徑亦有密切之互動關係,目前我們在細胞核內 基因轉錄因子的功能調控及細胞生長及細胞死亡等領域已有一些新穎有趣的發 現。例如:我們已知道 c-Jun 可藉由與 Sp1 的交互作用來活化 12(S)-lipoxygenase 基因表現,在這過程中 Sp1 扮演著“攜帶者”的角色,把 c-Jun 帶至調控基因之 啟動子處來達到基因活化的功能。在 B 型肝炎感染導致肝癌機制上,我們已知 道 pre-S 表面抗原變異蛋白可導致促肝細胞聚集增生之內質網壓力(ER stress)訊 息傳遞。 為了達成研究的目標,我們以過去的研究成果為基礎,分別成立三項分項計 畫。第一分項計畫乃基於過去我們在 12(S)-lipoxygenase 基因表現調控之經驗, 將進一步探討轉錄因子交互作用在基因轉錄調控所扮演的角色功能,同時也探討 磷酸化/去磷酸化對轉錄因子功能的影響。本分項包括探討 c-Jun 與 Sp1 交互作用 之分子機制,維他命 D 受體與 Sp1 交互作用活化 p27KIP1 基因的轉錄及 Sp1 在 Mrp 基因表現所扮演的角色。第二分項將著眼在台灣四種重要癌症訊息傳遞新機 制的探討。在膀胱癌的研究,將以 DOR1 及 RON 活化之訊息傳遞為重點;在大 腸癌的研究,將探討 Eps8 的訊息傳遞路徑,尋找 Ep8 訊息所調控的新基因產物; 在肺癌的研究,將探討 stat-3 活化導致癌化的新機制;在肝癌的研究上,將進一 步探討 B 型肝炎 pre-S 表面變異蛋白的訊息傳遞路徑。在第三分項計畫,則著眼 在探討細胞內抗細胞凋亡的機制及其訊息傳遞。在子宮頸癌,探討 caspase3 活化 所導致細胞生長的機制,並探討 FAK 蛋白分解機制以及細胞經何種機制來抗 1 FAK 分解所引發的細胞凋亡,並且解讀 T 細胞免於 Fas 訊號的毒殺,以及鋰如 何抑制免疫細胞的凋亡。在過去四年執行計畫期間,各分項的主要代表性發現如 下: 1) 在分項計畫(一),我們發現 Sp1 的去乙醯化在 12(S)-lipoxygenase 及 p21WAF1/CIP1 的基因表現調控,扮演一個重要角色。 2) 在分項計畫(二),我們發現 B 型肝炎病毒 pre-S2 mutant 可以促進肝細胞生長 及導致肝癌的形成。 3) 在分項計畫(三),我們釐清 caspase 2 在細胞凋亡所扮演的角色。 我們總共發表 59 篇 SCI 研究論文,其中有 36 篇發表在 IF>5 的學術期刊。 我們所得到的結果對於一些癌症產生的分子機制解析有相當的助益。另外在這一 計畫的推動過程,我們延攬 7 位研究助理教授參與以貢獻本計畫的進展,提供年 輕學者發展自己研究工作的機會。 配合各分項計畫的執行需求,我們在成大已建立一個研究細胞訊息傳遞的核 心設施,包括蛋白質體學、基因微陣列分析、光學影像儀器等設施,協助計畫成 員及成大其他同仁執行追求學術卓越研究。 1. Executive Summary (in English) The arrival of the post-genomic era in the twenty first century brings new challenges on biotechnological development and personnel education. The way we response to these challenges, determines the outcome of biomedical research and biomedical education in Southern Taiwan. We firmly believe that the solution lies in good biomedical education that must be backed up by strong research capabilities. Therefore, we integrated a group of researchers, mostly from NCKU, others from, National Sun Yat-Sen University and National Health Research Institutes. Among them are internationally renowned scientists and young researchers of great potentials to tackle this problem. The study of cellular signaling pathways and gene regulation was our main shaft. Instead of looking at individual signaling pathways (single dimensional studies), we conducted our studies from a multi-dimensional prospective. Through the study of “new mechanisms”, in search of “new genes”, we hoped to discover “new functions”-a pursuit of Research Excellency. We applied the same philosophy to tackle the four prevailing cancers in Taiwan that threaten the well beings of many of our countrymen. We also studied their pathology at the molecular level from a multi-dimensional prospective. Gene expression is regulated through intracellular signal transduction upon the stimulation of growth factors, hormones and other stimulants. The intracellular signal transduction pathways are dynamic and may cross talk with each other within the cell. We have made several interesting observations during our pervious research. We 2 discovered that direct cooperative interaction between c-Jun and Sp1 activates 12(S)-lipoxygenase expression, while Sp1 had a novel function as an anchor protein to recruit transcription factor c-Jun to the GC-rich box-containing gene promoter. In the studies of HBV-related hepato-cellular carcinogenesis, we found that the mutated pre-S proteins of the hepatitis B viral surface antigen were commonly present in chronic hepatitis B viral infected liver tissues. The pre-S mutants may cause down-regulation of small HbsAg in endoplasmic reticulum (ER) and thus results in ER stress. In the study of cervical carcinogenesis, we have found that activation of caspase-3 causes the augmentation of cancer cell growth instead of triggering apoptosis. These novel findings prompted the incubation of this proposal. Based on our previous research on gene regulation of 12(S)-lipoxygenase, we further studied the molecular mechanism of transcription factor activation, and the functional role of the post-translational modifications of Sp1 and c-Jun in gene expression in Sub-project (I). We also focused on the molecular mechanism of the interaction between c-Jun and Sp1, and transcriptional activation by the nuclear receptor/Sp1 complex in the gene activation of p27KIP1, and the cooperative regulation of the MRP gene by Sp1 and associated transcription factors. In Sub-project (II), novel mechanisms of signal transduction of four major cancers prevalent in Taiwan will be studied. We studied the signal transduction mediated through the activation of DOR1 and RON in bladder cancer, the signal transduction pathway induced by Eps8 activation in colon cancer and the role of Stat-3 activation in tumorigenesis of lung cancer. The signal transduction of ER stress induced by mutated pre-S of HbsAg in hepato-carcinoma was another major focus. In Sub-project (III), novel signal transduction mechanisms that mediate anti-apoptotic effects in patho-biology were focused. Mechanisms about the anti-apoptotic effects of caspase-3 activation, resistance to Fas/Fas L cytotoxicity, FAK protein degradation, and the anti-apoptotic mechanism of lithium in immune cells were studied. Our major representative findings in this project are as follows: 1) In Sub-project 1, we found that the deacetylation of Sp1 plays a functional role in gene regulation of 12(S)-lipoxygenase and p21WAF1/CIP1. 2) In Sub-project 2, we found that hepatitis B virus pre-S2 mutant upregulates cell proliferation of hepatocytes and tumorigenesis. 3) In Sub-project 3, we delineated the functional role of caspase 2 in apoptosis. We totally publish 59 SCI papers. Among them, 36 papers are published in several top journals (IF>5). Our research results contributes to the study of transcription factors-regulated gene regulation and signal transduction in cell growth regulation and carcinogenesis. Our another achievement is to recruit seven Research Associate Professors to participate the research in this project. 3 We have established the core laboratories at NCKU, in the past four years, which are essential for the success of this project. Deployment of larger instruments such as mass spectrometer, confocal microscope, fluorescence and chemiluminescence analyzers together with biochips allow the researchers involved in this project to compete internationally and pursuit the research excellency. 2. General Description The normal cellular function is under a sophisticated regulation network, so called “signal transduction”, to support the integrity of the system. When cellular growth control is abnormal, for example, the cell continuously grows until a tumor is formed which may damage the neighboring tissue and cause the organism to die. In addition, when a cell should go to apoptosis but does not, its presence may block the function of the neighboring cells and the whole tissue. Thus, to continuously perform normal cellular function, a cell needs to be cooperatively regulated by thousands of signal transduction processes within itself. Furthermore, the signal transmission is dynamic and cross-talk may occur within the cell. Therefore, it is also necessary for scientists to work with cross-talk in the research field of signal transduction. We proposed this project to integrate into a single research team all the intelligent scientists working in this field in southern Taiwan. So far, our team has been involved extensively in signal transduction and gene regulation research and has provided major contributions to the field. Among them only two of the more significant discoveries are mentioned here. First, in the study of how c-Jun and Sp1 work cooperatively in the activation of 12(S)-lipoxygenase expression, we discovered a novel function of Sp1 as a carrier to bring the transcription factor c-Jun to the GC-rich box-containing gene promoter. This is amongst the first few discoveries of such a novel transcriptional factor function. Second, in the studies of HBV-related hepato-carcinogenesis, we found that the mutated pre-S proteins of the hepatitis B viral surface antigen are commonly present in liver tissues of chronic hepatitis B viral infection, and the pre-S mutants may result in the down-regulation of small HbsAg in endoplasmic reticulum (ER) resulting in ER stress. Through intimate contact and intergration in this project, we addressed, at the molecular level, the tumorigenesis of the most important cancers in Taiwan. Also, we were also able to provide knowledge about the regulation of transcriptional factors in mediating gene expression and signal transduction in growth and apoptosis control. We divided this proposal, into three sub-proposals; (I) functional interaction of transcription factors in gene expression; (II) novel mechanisms of signal transduction of four cancers prevalent in Taiwan; and (III) studies of signal transduction mechanisms that contribute to cell survival. 4 3. Objectives Specifically, our aims, which were actualized by three subprojects, were to: 1) Elucidate functional interactions of transcription factors in gene expression regulation; 2) Study receptor tyrosine kinases signaling through Stat3/Eps8 in human cancer; and 3) Elucidate novel signal transduction mechanisms that mediate apoptosis or anti-apoptotic effects in patho-biology. 4. Interface and Integration between Overall and Sub-Projects The study of cellular signaling pathways and gene regulation is our main shaft in this project. Instead of looking at individual signaling pathways (single dimensional studies), we conducted our studies from a multi-dimentional prospective. Through the study of “new mechanism”, in search of “new genes”, we hoped to discover “new functions”. In order to improve the research infrastructure in the NCKU medical research center and form a technical support base for the whole project, we established six core laboratories in Overall project. They are (1) Mass Spectrometry (2) Microscopic Facility (3) Inducible Gene Expression (4) Functional Genomics and (5) Structural Biology. 5. Project Dr. W.C.Chang was responsible for the project management. In order to achieve our goals, the following strategies were reinforced. 1) Integration:There were frequent, informal intra-subproject interactions among the PIs. A formal progress report meeting for each subprojects was held once every 2~3 months. And the progress report for the whole project was held once every 5~6 months. Through the informal and formal meetings, we discussed about the technical help, insight sharing and discussion on possible relationship with their own projects. 2) Quality control:In order to guarantee success and minimize unnecessary waste of efforts, we have invited four distinguished scientists, three from abroad and one 5 local scientist to form an External Advisor Committee to oversee our research progress annually. They were responsible for critical appraisal of our research directions and results, and give important recommendations. The External Advisor Committee meeting was held once a year. 6. Describe in detail the approaches and methodologies to implement the research works CORE FACILITY Ⅰ: Proteomics Research Core Laboratory (PRCL) (Responsible Investigator: Pao-Chi Liao) Objective: To provide the following services: (1) Offer general courses such as, “Introduction to Proteomics” and “Laboratory for Proteomics Methods”. (2) Short (3-day) training courses for 2-D gel electrophoresis (2D-GE) (3) 2-D gel electrophoresis (4) Protein MW measurement/confirmation (5) Protein identification by mass specgtrometry (MS) Major instrumentations: (1) Six sets 2-D gel electrophoresis (two sets with multiple-gel capability) (2) Applied Biosystems DE-PRO MALDI-TOF mass spectrometer (3) Finnigan LCQ liquid chromatography-mass spectrometer (LC-MS) (4) Applied Biosystems QSTAR LC-MS with o-MALDI (funded by NSC) Work completed from 2002.4.1 to 2006.3.31: (1) Proteomics Research Core Laboratory (PRCL) has provided the following service to core lab users: (numbers in red: MOE project users) 服務項目 單位 2003 2003年 501 (139) 21(21) 68 (34) 2004年 2005年 2006.3 2002.9-2006.3 蛋白質二維凝膠電泳自行操作 人 蛋白質二維凝膠電泳委託操作 次 蛋白質或胜肽分子量測定 次 0 蛋白質身份鑑定 次 0 蛋白質二維凝膠電泳課程 次 22 (6) 成大暑期生物技術課程 人 0 20 16 26 0 52 南部科學園區產學協會生物技術課程 人 0 0 17 21 0 38 43 (0) 383 (73) 145 (38) 21 (13) 1093 (263) 88 (78) 314 (248) 103 (89) 34 (26) 111 25 (16) 2 (0) 18 (16) (106) 231 64 (45) 198 (147) 49 (40) (210) 56 (18) 22 (3) 12 (12) 0 (0) 6 156 (138) 542 (442) 112 (39) (2) PRCL has set up a web page (http://proteomics.med.ncku.edu.tw) to facilitate the promotion and operation of the services provided by the core lab. (3) Two full time operators have been employed for the operation of the core laboratory. (4) A fee structure has been implemented for service charges, as posted on the core lab web page. CORE FACILITY II: Time-lapse video microscopy/Biological imaging systems (Responsible investigator: Tzeng-Horng Leu/Meng-Ru Shen) Objective: The main purpose of this core is to provide instrumentation support of (1) time-lapse video microscopy and (2) phosphoimage analysis and (3) biological imaging systems for researchers in the MOE Program for Promoting Academic Excellence of Universities. (1) Time-lapse video microscopy: The Leica AS MDW system is purchased and set up for live cell imaging. All components like camera, shutters, piezo z-positoner and monochromator are fully integrated and optimized for light efficiency and acquisition speed in this system. Even fast cell dynamics can be recorded in 4D. This instrument will provide recording of intracellular proteins/organelle translocation as well as long-time observation of cellular movement. It was set up and started to provide service since the July of 2003. This instrument provides recording of intracellular proteins/organelle translocation as well as long-time observation of cellular movement. In this year, there are 34 times (calculated from Dec. 26, 2004 to Dec. 30, 2005) people have utilized this machine in their research in this year. This has added up total 70 times that have been used since it was established in the July of 2003. (2) Phosphoimage analysis: An FLA-5000 imaging system (Fujifilm) and a LAS-1000plus system (Fujifilm) are purchased for analyzing images of radioisotope and fluorescent respectively. The machines were set up in Dec. 2002 and are providing service since then. (3) Biological imaging systems: We have set up a core laboratory of optical imaging with the financial support from MOE Program for Promoting Academic Excellence of Universities and Center for Bioscience and Biotechnology, National Cheng Kung University. This core laboratory is well equipped with (1) a new generation of confocal microscope for live cell 7 imaging system; (2) an atomic force microscope (AFM) coupled with a confocal laser scanning biological microscope; (3) an inverted research microscope coupled with high speed cooling CCD and fluorescence illuminators. The function of the core laboratory is to analyze the dynamic processes in living cells, such as cytoskeleton dynamics, secretory membrane trafficking, cellular interactions, chromatin dynamics, intracellular pH and calcium measurement with simultaneously electrophysilogical recording. The training courses of advanced bioimaging were held every three months. In addition, this core laboratory has serviced 120 hr/month for the users. CORE FACILITY III: Multiple inducible gene expression cell model laboratory (Responsible Investigator: Hsiao-Sheng Liu) Objective: The objectives of this core facility are to assist PIs in each subproject to utilize our inducible systems to regulate the genes of interest. Facility: GenePulser XcellTM (BioRad) is an electroporator, which is extremely powerful for DNA, RNA and protein delivery. Accomplishments and service: For training more people to use this electroporator, we hold a lecture in 2004, and 2005. A total of over fifty people attended. In each year, more than 60 services are made during the four year period. In this core facility, the following inducible systems are available and servied: 1. the lactose repressor system (Lac system); 2. the insect hormone ecdysone-dependent expression system (Ecd system); 3. the tetracycline-dependent expression system (Tet system). Furthermore, a Cre/lox P system has been used to construct Tet inducible system. This system further simplifies the procedure of cloning. At least three papers have been published using this instrument. Paper published: 1. Jih I Chuang, Tsuey Y Chang and Hsiao S Liu. 2003. Glutathione depletion-induced apoptosis of Ha-ras-transformed NIH3T3 cells can be prevented by melatonin. Oncogene 22, 1349–1357. (卓越計畫) 2. Ya-Shih Tseng, Ching-Cherng Tzeng, Allen Wen-Hsiang Chiu, Chia-Hsiang Lin, Shen-Jeu Won, I-Chien Wu, and Hsiao-Sheng Liu, 2003. Ha-ras overexpression mediated cell apoptosis in the presence of 5-fluorouracil. Exp. Cell Res., 288, 403–414. 3. Tsuey-Yu Chang, Wen-Jiuan Tsai, Chao-Kai Chou, Nan-Haw Chow, Tzeng-Horng 8 Leu, Hsiao-Sheng Liu. 2003. Identifying the factors and signal pathways necessary for anchorage-independent growth of Ha-ras oncogene-transformed NIH/3T3 cells. Life Sci., 73, 1265-1274. (卓越計畫) 4. Yeh, H. H., Lai, W. W., Chen, H. H. W., Hsiao S Liu *, and Su, W. C.* 2006. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene [Epub ahead of print]. (*: corresponding author). (卓越計畫) 5. Tseng, Y. S., Tzeng, C. C., Huang, C. Y. F., Chen, P. H., Chiu, Allen W. H., Hsu, P. Y., Huang G. C., Wang, Y. C., and Hsiao S Liu. 2005. Aurora-A overexpression associates with Ha-ras codon-12 mutation and blackfoot disease endemic area in bladder cancer. Cancer Lett., [Epub ahead of print] 6. Chou, C. K., Liang, K. H., Tzeng, C. C., Huang, G. C., Chuang, J. I., Chang, T. Y., and Hsiao S Liu. 2006. Dominant negative Rac1 suppresses Ras-induced apoptosis possibly through activation of NFκB in Ha-ras oncogene-transformed NIH/3T3 cells. Life Sci., 78, 1823-9. CORE FACILITY IV: DNA Microarray (Responsible Investigator: Hsiao-Sheng Liu) Objectives: We missions are to support every PI whether he is in the MOE program or not, to better use this core facility to increase the academic excellencies in their research domains. Facilities: The basic equipments for running this core facility have been purchased and implemented. Supported by the MOE program, the equipments include a deep freezer for preserving the cDNA clones, two PCR machines, one fluorescence scanner with two laser beams, a server computer and a PC for the implantation of yearly-subscribed microarray analysis software. Currently, the arrayer and the scanner are located at our Medical school (building room 82-1039) (the microarray center). Accomplishments and service: So far we have a collection of 2,600 human cDNA clones and 228 mosquito cDNA clones in this core facility. Most of the clones selected for further analysis have been re-sequenced and re-annotated during the past year. The total numbers of services exceed 122 in 2004 and exceed 69 in 2005. So far, we have produced four versions of chips, and the chip 4 “oncogene and kinase” chip is under active service now. Ras signal pathway chip is our version V chip and starts being used now. Cell cycle and apoptosis chip has been designed, and will be released in the future. To encourage 9 more people to use our chips and facility, we had held three lectures to introduce our chip service in 2005 and one in 2006. The detailed information regarding the microarray core facility is on the website http://140.116.247.183/smallfat/website1/index2.htm. Paper published: 1. Yu-Chiao Hsu, Ching-Cherng Tzeng, Da-En Cheng, Hsuan-Heng Yeh, Wen-Ten Wong, Ya-Shih Tseng, Chen-Yun Yeh, Tsuey-Yu Chang, Ya-Ping Lin, Ying-Ray Lee, Nan-Haw Chow, and Hsiao-Sheng Liu. 2006. Ras down-regulates the metastasis suppressor RECK through up-regulation of RbAp46, which complexes with HDAC1. (Submission to Cancer Research) (卓越計畫) 2. Chen Y. Yeh, Nan H. Chow, Tsuey Y. Chang, Hui C. Shen, Jyh W. Shin, Jung H. Chiang, S.M. Vincent Tseng, and Hsiao-Sheng Liu. 2006. The importance of Axl and PDGFR- in c-Met-associated bladder carcinogenesis. (Submission to Cancer Research) (卓越計畫) CORE FACILITY Ⅴ: Structural Biology Core A: Lab for NMR and Protein Expression (Responsible Investigator: Woei-Jer Chuang) Objective: The main purpose of this core is to provide instrumentation support and service to MOE investigators. The aims of this structural core lab are to: (1) determine the 3D structures of proteins involving this project; (2) produce large quantities of proteins for NMR studies; and (4) model protein structure and analyze chemical and physical properties of proteins involving this project. Facilities and equipments: Server: Sun Fire 6800 Workstation: SGI Octane Fuel Softwares: SRS7, EMBOSS, Artemis, InsightII, Xplor, CNS, Seqfold, Homology, Modeller, Consensus, and Autodock Fermentors: NBS Celligen (protein production in CHO cell) NBS Bioflo 101 (mass protein production in Pichia pastoris) NBS Bioflo 101 (mass protein production in E. coli) Rhoche ProteoMaster: mass protein production in cell free expression system. The Core insures that these instruments are maintained in good working condition. 10 Results: A number of important results have been achieved in the past year. (1) The facility and equipments have been installed and the services were provided starting from November, 2002. (2) >20 papers were published by using this core facility. (3) Four papers were published and sponsored by this grant. 1. Liu, P.-P., Chen, Y.-C, Li, C., Hsieh, Y.-H. Chen, S.-W., Chen, S.-H., Jeng, W.-Y., and Chuang, W.-J. (2002) “Solution Structure of the DNA-Binding Domain of Interleukin Enhancer Binding Factor 1 (FOXK1a)“, Proteins, 49, 543-553. 2. Chuang, W.-J. , Yeh, I-Ju, Hsieh, Y.-H. Chen, S.-W., Liu, P.-P., Jeng, W.-Y. (2002) “1H, 15N and 13C Resonance Assignments for the DNA-Binding Domain of Myocyte Nuclear Factor (Foxk1)”, J. of Biomolecular NMR, 24, 75-76. 3. Wang, C.-C., Chen, J.-H., Yin, S.-H., and Chuang, W.-J. (2006) “Predicting the Redox State and Secondary Structure of Cysteine Residues in Proteins Using NMR Chemical Shifts”, Proteins, 63, 219-226. 4. Wang, C.-C., Chen, J.-H., Lai, W.-C., and Chuang, W.-J. (2006) “2DCSi: Determining Secondary Structures and Redox States of Proteins Using 2D Cluster Analysis of NMR Chemical Shifts”, Bioinformatics, in revision. B: Laboratory of Combinatorial Chemistry and Peptides Synthesis (Responsible Investigator: Wai-Ming Kan) Objective: The main purpose of this core is to provide instrumentation support and service to MOE investigators. The aims of this structural core lab are to: (1) Peptide and Phosphopeptide Synthesis (2) Combinatorial Peptide/Small Molecule Libraries Preparation -- for the identification of substrate selectivity, kinase recognition sequences, and enzyme inhibitors or receptor ligands. Facilities and equipments: Organic Synthesizers: (1) EYELA solid phase organic synthesizer CCS-1200V (2) EYELA ChemiStation Model PPW-2000 Workstation: SGI O2+ Softwares: Cerius2 C2•Visualizer; C2•DMol3 Interface; C2•DMol3-Molecular; C2•Dynamics; C2•Minimizer; C2•MOPAC Interface and MOPAC program; C2•Open Force Field The Core insures that these instruments are maintained in good working condition. 11 Results: 1. Preparation of commercially unavailable prostacyclin antagonist, 3-[4-(4, 5-dihydro-1H-imidazol-2-ylamino) phenyl]-1-(4- morpholino-phenyl) propan-1 -one (US6417186B1), and their respective conformational restricted derivatives. 2. Preparation of commercially unavailable selective GSK3 inhibitor AR-A014418. 3. Preparation of non-, mono- and di-phosphorylated Syk peptides for the study of the effect of phosphorylation on the structure of the peptides. 4. Preparation of commercially unavailable cell permeable TAT-V1-1 peptide. 5. Preparation of commercially unavailable RO1138452. Sub-project (I) Functional interaction of transcription factors in gene expression (Principal Investigator: Wen-Chang Chang) Our previous studies indicate a novel function of Sp1 that could be as an anchor protein to recruit other transcription factors c-Jun, and deacetylation of Sp1 plays a functional role in 12(S)-lipoxygenase gene expression (Fig. 1). In the past year of this Sub-project, we focused our studies on the functional role of post-translational modification of c-Jun, VDR and Sp1 in the transcriptional regulation of cellular genes. In Sub-project I, we found that calcineurin-mediated dephosphorylation of c-Jun C-terminus was required for c-Jun/Sp1 interaction in phorphol ester-induced 12(S)-lipoxygenase gene expression. We also found that Sp1 could be acetylated, and the acetylated Sp1 could enhance the 12(S)-lipoxygenase gene expression. These results indicated that dephosphorylation of c-Jun C-terminus and acetylation of Sp1 play a pivotal role in 12(S)-lipoxygenase gene transcription. In Sub-project Ⅱ, we identified a number of potential genes that could be regulated by VDR/Sp1 complex. For example, vitamin D3 repressed the expression of Skp2, an F box protein that is involved in the degradation of p27Kip1. We also found that dephosphorylation of VDR enhanced its interaction with Sp1. Based on these findings, we focused our studies on the effects of post-translational modifications(acetylation and sumoylation)of Sp1 on its binding with c-Jun and VDR. PMA cytoplasm c-Jun nucleus c-Jun HDAC1 HDAC1 c-Jun Acetyl Sp1 H3 Step 1 p300 c-Jun Sp1 H3 Acetyl Step 2 p300 c-Jun Sp1 H3Acetyl Step 3 12 Figure 1. A scheme illustrating the mechanistic model of PMA-induced activation of the 12(S)-lipoxygenase transcription (Hung, J. J. et al., Mol. Cell. Biol. 2006) I-1-1: Calcineurin-mediated dephosphorylation of c-Jun C-terminus is required for c-Jun/Sp1 interaction in phorbol ester-induced 12(S)-lipoxygenase gene expression (Ben-Kuen Chen and Wen-Chang Chang) The interaction c-Jun/Sp1 is essential for growth factor- and for phorbol 12-myristate 13-acetate (PMA)-induced genes expression, including human 12(S)-lipoxygenase, keratin 16, cytosolic phospholipase A2, p21WAF1/CIP1 and neuronal nicotinic acetylcholine receptor 4. Here, we examined the mechanism underlying the PMA-induced regulation on the interaction between c-Jun and Sp1. We found that treatment of cells with PMA induced a dephosphorylation at the C-terminus of c-Jun and a concomitant inhibition of calcineurin (PP2B) that has been demonstrated by using cyclosporin A and PP2B small interfering RNA resulting in reduced PMA-induced gene expression as well as the c-Jun/Sp1 interaction. The c-Jun mutant TAM-67-M3A, which contains three substitute alanines at Thr-231, Ser-243, and Ser-249 when compared to TAM-67, is bound more efficaciously with Sp1 and is about twice as efficacious as TAM-67 in inhibiting the PMA-induced activation of the 12(S)-lipoxygenase promoter. Importantly, PP2B not only dephosphorylates the phospho-TAM-67 in an in vitro assay but also interacts with c-Jun in PMA-treated cells. PMA stimulates the association of the PP2B/c-Jun/Sp1 complex with the promoter. These findings indicate the dephosphorylation of c-Jun C-terminus that is required for the c-Jun/Sp1 interaction and reveal that PP2B plays an important role in regulating c-Jun/Sp1 interaction in PMA-induced gene expression. To investigate domains involved in the regulation of c-Jun and PP2B interaction, immunoprecipitation and FRET assays were used. Both calmodulin and calcineurin B domains of PP2B were involved in the binding with c-Jun. Since PMA also induced the interaction between c-Jun and PP2B, we want to analyze the function and mechanism involved in the regulation of c-Jun/PP2B complex. PP2B was sumoylated both in PMA-treated cells and in an in vitro enzymatic assay. These results indicated that PMA induced the interaction between c-Jun and PP2B may be through the posttranslational protein modification. In addition, PMA-induced PP2B sumoylation also indicated the possibility of novel function of PP2B in the gene expression. I-1-2: Role of Sp1 in Transcriptional Regulation of 12(S)-Lipoxygenase (Jan-Jong Hung and Wen-Chang Chang) a. Sp1 is a basic transcriptional factor, which binds to the GC-rich region in the promoter of target gene. It is involved in transcription of numerous genes by 13 recruiting other transcriptional factors to the promoters of target genes. In this study, we found in vivo and in vitro that Hsp90 was recruited to the GC-rich region of 12(S)-lipoxygenase promoter by interaction with Sp1 in A431 cells by employing DNA affinity immunoprecipitation assay and chromatin immunoprecipitation assay. When Hsp90 was inhibited by geldanamycin (GA), a specific inhibitor of Hsp90 family, or by siRNA of Hsp90 to block its activity or to knockdown protein level respectively, the luciferase activity driven by the 12(S)-lipoxygenase promoter, and both of mRNA and protein levels of 12(S)-lipoxygenase all were reduced significantly in cells. In addition, the effect of GA was abolished when the Sp1-binding sites of 12(S)-lipoxygenase were mutated in A431 cells. Interestingly, binding of Sp1 to the 12(S)-lipoxygenase promoter was also decreased upon GA treatment in cells. In conclusion, our results indicated that Sp1 interacted with Hsp90 to recruit it to the promoter of 12(S)-lipoxygenase, and then to regulate the gene transcription by affecting the binding ability of Sp1 to the promoters. b. We previous reported that Sp1 recruits c-Jun to the promoter of 12(S)-lipoxygenase in 12-myristate 13-acetate treated cells. We now show that Sp1 was constitutively acetylated that recruited HDAC1 to the Sp1/cJun complex when cells were exposed to PMA (3 h). Prolonged stimulation of the cells with PMA (9 h), however, caused the dissociation of HDAC1 and the deacetylation of Sp1 with the latter being able to recruit p300 that in turn caused the acetylation and dissociation of histone 3, thus enhancing the expression of 12(S)-lipoxygenase. We also overexpressed Sp1 mutant (K703/A; lacking acetylation sites) in the cell and found that cells recruited more p300 and expressed more 12(S)-lipoxygenase. Taken together, our results indicated that Sp1 recruits HDAC1 together with c-Jun to gene promoter, followed by deacetylation of Sp1 upon PMA treatment. The p300 is then recruited to gene promoter through the interaction with deacetylated Sp1 to acetylate histone 3, leading to enhancement of the expression of 12(S)-lipoxygenase. I-2: Molecular mechanism of interaction between vitamin D receptor and Sp1 in gene regulation (Wen-Chun Hung) The steroid hormone vitamin D3 plays an important role in the regulation of numerous physiological and cellular processes including calcium and phosphorous homeostasis, cell growth, differentiation and apoptosis. The biological effect of vitamin D3 is mainly mediated via the vitamin D3 receptor (VDR). Binding of vitamin D3 to VDR promotes the interaction between VDR and retinoid X receptor (RXR) and enhances the formation of VDR/RXR heterodimer. The heterodimer then binds to the vitamin D3 response element (VDRE) in the promoter of target genes to stimulate gene transcription. In addition to classic genomic action, recent studies demonstrate 14 that vitamin D3 may activate several intracellular signaling pathways such as extracellular signal-regulated kinases (ERKs), c-Src kinase and phospholipases in a non-genomic fashion. Recent studies also demonstrate that steroid hormones may activate the expression target genes in which the promoter regions lack steroid receptor response elements. It is possible that steroid hormone/receptor complexes, instead of directly binding to DNA, may interact with transcription factors to modulate gene expression. We try to elucidate whether VDR may interact with Sp1 transcription factor and regulate gene transcription via GC-rich Sp1 sites. A number of conclusions are obtained in this four-year study: First, we demonstrate that vitamin D receptor (VDR) may physically interact with Sp1 transcription factor. GST-pull down assay indicated that Sp1 interacted with VDR via its N-terminal (a.a. 1-283) and C-terminal (a.a. 611-778) domain. In addition, our results demonstrate that the VDR/Sp1 complex binds to the Sp1 binding sites corresponding to the sequence –544 to -512 bp of human p27Kip1 promoter to trigger gene expression (1). Second, microarray analysis suggests that transcription of a number of genes can be positively or negatively regulated by the VDR/Sp1 complex. For example, expression of p27Kip1 and CD14 (2) is activated by the VDR/Sp1 complex via the Sp1 sites in the promoter. On the contrary, expression of Cks1 and Skp2 is repressed by this complex via a Sp1-dependent manner. Third, we address whether the VDR/Sp1 complex activates gene expression via the transactivation domain of VDR and find that the AF-2 activation domain of VDR is necessary for the activation of p27Kip1 by the VDR/Sp1 complex (3). Therefore, Sp1 functions as an anchor protein and VDR functions as the transactivation component in the VDR/Sp1 complex. Fourth, the VDR/Sp1 complex may suppress gene transcription via recruitment of histone deacetylases (HDACs) (4). Proteomic analysis is now performing to address the other co-repressors in this repression complex. Fifth, our recent data indicate that VDR can be sumoylated. We find that K103 is a major sumoylation site for VDR and sumoylation may enhance the transcription activity of this nuclear receptor (5). Collectively, our study elucidates a molecular mechanism by which VDR regulates gene expression and provides new insights for the understanding of the biological action of vitamin D3 in vivo. References 1. Huang YC, Chen JY, and Hung WC. (2004) Vitamin D3/Sp1 complex is required for the induction of p27Kip1 by vitamin D3. Oncogene 23, 4856-4861. 15 2. Chen JY, Huang YC, and Hung WC. (2006) Regulation of CD14 expression by the VDR/Sp1 complex. (in preparation) 3. Cheng HT, Chen JY, Huang YC, and Hung WC. (2006) Functional role of VDR in the activation of p27Kip1 by the VDR/Sp1 complex. J. Cell. Biochem. (in press) 4. Huang YC, and Hung WC. (2006) Vitamin D3 transcriptionally represses p45Skp2 expression via Sp1 sites in human prostate cancer cells. (revised) 5. Tsai NG, and Hung WC. (2006) Sumoylation of VDR modulates its transcription activity. (in preparation) List of Research Publications: 1) Yi-Wen Liu, Hui-Ping Tseng, Lei-Chin Chen, Ben-Kuen Chen, and Wen-Chang Chang. (2003) Functional cooperation of simian virus 40 promoter factor 1 and CCAAT/enhancer-binding protein β and δ in lipopolysaccharide-induced gene activation of IL-10 in mouse macrophages. J. Immunol. 171, 821-828. 2) Ying-Nai Wang and Wen-Chang Chang. (2003) Induction of the diseaseassociated keratin 16 gene expression by epidermal growth factor is regulated through cooperation of transcription factors Sp1 and c-Jun. J. Biol. Chem. 278, 45848- 45857. 3) Wen-Chang Chang. (2003) Cell signaling and gene regulation of human 12(S)lipoxygenase expression. Prostaglandins Other Lipid Mediat. (Invited Review) 71, 277-285. 4) Te-Hsiu Lee, Hui-Chiu Chang, Lea-Yea Chuang, and Wen-Chun Hung. (2003) Involvement of PKA and Sp1 in the induction of p27Kip1 by tamoxifen. Biochem. Pharmacol. 66, 371-377. 5) Yu-Chun Huang, Jing-Yi Chen, and Wen-Chun Hung. (2004) Vitamin D3 receptor/ Sp1 complex is required for the induction of p27Kip1 expression by vitamin D3. Oncogene 23, 4856-4861. 6) Lei-Chin Chen, Ben-Kuen Chen, Jia-Ming Chang, and Wen-Chang Chang. (2004) Essential role of c-Jun induction and coactivator p300 in epidermal growth factor-induced gene expression of cyclooxygenase-2 in human epidermoid A431 cells. Biochim. Biophys. Acta (Mol. Cell Biol. L.) 1683, 38-48. 7) Lei-Chin Chen, Ben-Kuen Chen, and Wen-Chang Chang. (2005) AP1-mediated cyclooxygenase-2 expression is independent of N-terminal phosphorylation of c-Jun. Mol. Pharmacol. 67, 2057-2069. 8) Ju-Ming Wang, Joseph T. Tseng, and Wen-Chang Chang. (2005) Induction of human NF-IL6β by epidermal growth factor is mediated through p38 signaling pathway and cAMP response element-binding protein activation in A431 cells. Mol. Biol. Cell 16, 3365-3376. 16 9) Shwu-Jen Tzeng, Wen-Chang Chang, and Jin-Ding Huang. (2005) Transcriptional regulation of the rat Mrp3 gene promoter by the specificity protein (Sp) family members and CCAAT/enhancer binding proteins. J. Biomed. Sci. 12, 741-761. 10) Wen-Chang Chang and Ben-Kuen Chen. (2005) Transcription factor Sp1 functions as an anchor protein in gene transcription of human 12(S)-lipoxygenase. Biochem. Biophys. Res. Commun. (Invited Review) 338, 117-121. 11) Jan-Jong Hung, Chih-Ying Wu, Pao-Chi Liao, and Wen-Chang Chang. (2005) Hsp90 recruited by Sp1 is important for transcription of 12(S)-lipoxygenase in A431 cells. J. Biol. Chem. 280, 36283-36292. 12) Ying-Nai Wang, Yung-Ju Chen, and Wen-Chang Chang. (2006) Activation of ERK signaling by epidermal growth factor mediates c-Jun activation and p300 recruitment in keratin 16 gene expression. Mol. Pharmacol. 69, 85-98. 13) Yi-Wen Liu, Chun-Chia Chen, Hui-Ping Tseng, and Wen-Chang Chang. (2006) Lipopolysaccharide-induced transcriptional activation of interleukin-10 is mediated by MAPK- and NF-κB-induced CCAAT/enhancer-binding protein δ in mouse macrophages. Cell. Signal. (in press) 14) Jan-Jong Hung, Yi-Ting Wang, and Wen-Chang Chang. (2006) Sp1 deacetylation induced by phorbol ester recruits p300 to activate 12(S)-lipoxygenase gene transcription. Mol. Cell. Biol. 26, 1770-1785. 15) Ju-Ming Wang, Chiung-Yuan Ko, Lei-Chin Chen, Wen-Lin Wang and Wen-Chang Chang. (2006) Functional role of NF-IL6β and its sumoylation and acetylation modifications in promoter activation of cyclooxygenase 2 in A431 cells. Nucleic Acids Res. 34, 217-231. 16) Hsuen-Tsen Cheng, Jing-Yi Chen, Yu-Chun Huang, Hui-Chiu Chang, and Wen-Chun Hung. (2006) Functional role of VDR in the activation of p27Kip1 by the VDR/Sp1 complex. J. Cell. Biochem. (in press) 17) Ming-Chuan Hsu, Hui-Chiu Chang, and Wen-Chun Hung. (2006) HER-2/neu represses the metastasis suppressor RECK via ERK and Sp transcription factors to promote cell invasion. J. Biol. Chem. 281, 4718-4725. 18) LunYu Kuo, Hui-Chiu Chang, Tzeng-Horng Leu, Ming-Chie Maa, and Wen-Chun Hung. (2006) Src oncogene activates MMP-2 expression via the ERK/Sp1 pathway. J. Cell. Physiol. 207, 729-734. 19) Ben-Kuen Chen, Chi-Chen Huang, Wei-Chiao Chang, Yung-Ju Chen, Ushio Kikkawa, and Wen-Chang Chang. (2006) Calcineurin-mediated dephosphorylation of c-Jun C-terminus regulates phorbol ester-induced c-Jun/Sp1 interaction. Mol. Biol. Cell (in revision) Sub-project (II) Studies of receptor tyrosine kinases signaling through Stat3/Eps8 17 in human cancers (Principal Investigators: Ih-Jen Su and Tzeng-Horng Leu) Receptor tyrosine kinases, such as EGF receptor family members and HGF receptor family members play important role of a variety of human cancer formation. Studies from us and many others indicated no single oncogenic protein is responsible for the tumor formation in different tissues. However, comparing the protein molecules activated or overexpressed in bladder cancer, colon cancer, lung cancer and liver cancer, as studied in this four-year project, several important proteins and/or pathways do come out to be important for the oncogenesis of these cancer. The summaries of our findings in the mechanisms of four major cancers in Taiwan are indicated below. II-1: The novel mechanisms of tumor stroma and cellular oncogenes in modulation of bladder carcinogenesis (Nan-Haw Chow and Hsiao-Sheng Liu) The extracellular matrix (ECM) of tissue stroma is known to influence the proliferation, differentiation, and morphogenesis of normal epithelial cells. The aberration of ECM contents, as well as its interaction with epithelial cells, may also impinge upon biological properties of cancer cells. The ECM-derived growth factors include hepatocyte growth factor (HGF), basic fibroblast growth factor and vascular endothelial growth factor. Our cohort study has demonstrated the clinical significance of HGF/c-met pathway in the progression of human bladder cancer. This project was designed to discover novel mechanism(s) involved in bladder carcinogenesis as well as the potential targets for cancer therapy. The major findings during this four-year project are summarized below. (a) The significance of RON receptor in the tumor progression of human bladder RON receptor tyrosine kinase belongs to c-met receptor family and is a specific cell-surface receptor for macrophage stimulating protein (MSP). Activation of MSP/RON has been demonstrated to induce in vitro epithelial cell migration, proliferation, and tumorigenicity or metastasis in vivo. With regard to huamn bladder cancer, we have disclosed the existence of MSP in the urine of bladder cancer patients (n = 8), and expression of MSP in 6 of 10 uroepithelial cell lines tested. Moreover, expression of wild-type RON was detected in 5 uroepithelial bladder cancer cell lines. In contrast, both UB47 and UB40 bladder cancer cell lines have a 147 bp deletion in the extracellular domain of RON receptor (RON), resulting in aberrant localization of RON in the cytoplasm. Overexpression of RON was revealed in 56 of 165 bladder cancer patients (33.9%), and the expression level positively relates to histological grading, muscle-invasion, tumor size 18 (p < 0.005) and overall patient survival (p < 0.0001). The expression status of Met in the same cohort was also examined. A total of 70 cases (42.4%) showed overexpression of Met, and the expression positively associates with patient survival (p = 0.015). Interestingly, co-expression of RON and Met showed prognostic importance in predicting patient survival compared to expression of single receptor or without receptor expression (p = 0.0003). In addition, in vitro experiments further prove that overexpression of RON induces the growth, migration, and anti-apoptosis of human bladder cancer cells. Constitutive phosphorylation was detected in cancer cells having either wild-type or mutant RON expression, and phosphorylation of RON was further enhanced by MSP. Take together, both clinicopathologic and in vitro studies indicate that MSP/RON pathway, as well as its interaction with HGF/Met, plays an important role in the progression of human bladder cancer. (b) Collaboration of RON and EGFR in tumorigenesis of human bladder Cross-talk between heterologous receptor tyrosine kinases has emerged as an important paradigm in the molecular pathogenesis of human cancer. A recent study reported that RON-related scattering activity and transformation seem to be mediated by transactivation of epidermal growth factor receptor (EGFR), but the mechanism underlying EGFR-dependent activation remains largely unknown. Using TSGH8301 human bladder cancer cell line, expressing both RON and EGFR receptors, and J82 cells, expressing EGFR only, we demonstrated that EGFR transactivates RON via direct association, even in the absence of ligand. EGFR kinase inhibitors efficiently inhibit the activation of RON and its related mitogenesis, migration, anti-apoptosis, and neoplastic transformation. As for clinical implication, co-expression of RON and EGFR was revealed in 26 of 78 (33.3%) bladder cancer patients, and was significantly correlated with tumor invasion (P < 0.05), risk of local recurrence (P = 0.0003), or decreased patient survival (P = 0.04). Important indicators in predicting patient survival were co-expression of RON and EGFR (P = 0.001) and tumor staging (P = 0.05). Taken together, these results of our study support the potential significance of cross-talk between RON and EGFR in vivo, and identify a novel ligand-independent activation of RON-related signaling by EGFR. The observation may shed additional light on the implication of EGFR inhibitors in cancer therapy. (c) The molecular basis for nuclear import of RON receptor in human bladder cancer cells We demonstrated that de-phosphorylated status of both wild-type and truncated RON are detected in the nuclei of cancer cells. As a result, nuclear translocalization of RON receptor may play an important role in the progression of bladder cancer. In 19 addition, translocalization of de-phosphorylated RON appears to be mediated by ligand-independent mechanism. The nuclear RON was co-localized with importin 1, importin 1 and de-phosphorylated EGFR. Nevertheless, MSP treatment induces phosphorylation of EGFR and dissociation from nuclear RON, implying a cross-talk between RON and EGFR. The siRNA experiments confirmed the importance of EGFR on nuclear translocalization of RON. In addition, collaboration of RON and EGFR contributes to RON-mediated biological effects in human bladder cancer cells. To verify the significance of nuclear localization signal (NLS) in the nuclear translocalization of RON, site-directed mutagenesis assay was performed on both Nand C-terminal NLSs of RON. Our preliminary results showed that R306Q at N-terminal and both R1388T and R1389T at C-terminal NLS of RON effectively suppress the nuclear translocalization, supporting a classical NLS-dependent mechanism. The suppression of proliferation and anti-apoptosis of NLS mutants suggests that nuclear RON might confer growth advantage for cancer cells. Given that heat shock proteins (HSPs) are chaperones in regulating stress-induced signaling and nuclear transport of cell cycle kinases, expression of HSPs was analyzed by immunoblotting on subcellular fractions. Levels of HSP70, HSP90 and heat shock factor 1 (HSF 1) were all increased in the nuclear fraction. The DAPA revealed that nuclear RON/EGFR complex binds to AP1-binding site of promoter, suggestive of a transcriptional regulation for nuclear RON. On the basis of findings described above, we hypothesize that RON receptor transduces two distinct signaling pathways. One is MSP-dependent activation of ERK/Elk and PI3K/Akt signaling pathways. The other is ligand-independent pathway, in which full-length of RON receptor co-localizes with EGFR, importin 1 and 1 into the nucleus. MSP seems to impose a higher affinity to membrane RON receptor and thus driving the dissociation of RON from EGFR in the nucleus. The nuclear RON/EGFR complex may bind to AP1 or SP1 binding site in modulation of gene expression. Whether these pathways are redundant or divergent in controlling of biological activities remains to be elucidated. Since co-expression of RON/Met also plays a role in the metastatic progression of bladder and breast cancers, interaction between EGFR and RON may play an important role in the progression of epithelial carcinogenesis. The key issues under intensive investigation include the mechanism underlying nuclear translocation of RON/EGFR complex, the roles of RON/EGFR as transcription factor, and its biological implication in epithelial carcinogenesis. II-2: Aberrant expression of signaling proteins in human colorectal tumors: Eps8 overexpression promotes colon cancer formation (Tzeng-Horng Leu) FAK and c-Src are two mutually interactive nonreceptor tyrosine kinases in 20 signal transduction. Overexpression and/or of FAK and c-Src is individually observed in a variety of human tumors, especially in colorectal cancer. However, the relationship of both proteins in the carcinogenesis of colon cancer is vague. The molecular mechanism to enhance and/or activate these two kinases in colon cancer was not known, neither. Our early studies have indicated a Src-regulated and -phosphorylated protein, Eps8, is a potential oncogene whose overexpression causes tumor formation in mice. Therefore, in this project, we want to address whether 1) FAK and Src cooperated in colorectal cancer; 2) Eps8 is participated in v-Src-mediated transformation; and 3) how Eps8/FAK/Src interact with each other in the promotion of colorectal cancer progression. The following is our major findings during this four-year project. (a) Parallel overexpression of FAK and Src in colorectal cancer To investigate the role of FAK and Src in the multistep colorectal carcinogensis simultaneously, we analyzed their expression in 60 paired cancer-normal mucosa specimens from colorectal cancer patients. Compared to normal mucosa, enhanced FAK and c-Src expression in tumor specimens (T/N > 2) was observed in 48.3 % (29/60) and 68.3 % (41/60) tumor samples respectively while no altered expression of p130Cas was detected. Interestingly, the expression levels of both proteins are parallel in these tumors. Furthermore, their coexpression is sensitive to histone deacetylase (HDAC) inhibitors, such as sodium butyrate and trichostatin A, indicating a common pathway in regulating their expression present in colon cancer cells. (b) Participation of p97Eps8 in Src-mediated transformation TSA is a well-known histone deacetylase inhibitor. When we treated IV5 cells with TSA, we observed the expression of Eps8 is significantly reduced that correlated well with the reduced growth rate of v-Src transformed cell IV5. The relationship between growth and Eps8 expression can be further demonstrated by introducing eps8 siRNA into IV5 cells resulting in significant growth inhibition in both culture dish and in mice. Furthermore, when p97Eps8 was introduced into active Src-expressed CE cells, it reversed the growth inhibition activity of TSA. Thus, p97Eps8 plays an important role in Src-mediated transformation. (c) Eps8 participates in colon tumorigenesis through Src and focal adhesion kinase activation The role of Eps8 in human carcinogenesis is still unclear. Within 62% of colorectal tumor specimens examined, greater than two fold enhancement of p97Eps8 expression as compared to their normal counterparts was observed, especially for 21 those from the advanced stage. For SW480 cells ectopically expressed Eps8, their increased proliferation in culture and tumor formation in nude mice bolster the importance of Eps8 in colon tumorigensis. Concurrently, Eps8 knock down by overexpressing eps8 siRNA results in growth inhibition of SW620 and WiDr cells. In agreement with the concomitant expression of Eps8 and FAK in tumor specimens, we prove that Eps8 could modulate the expression of FAK. Remarkably, overexpression of FAK could relieve the growth retardation mediated by Eps8 attenuation. Furthermore, Eps8 associates with FAK and Src and mediates their activation, which in turn increases the phosphorylation of Shc and activates ERK. Notably, the abrogation of motility in SW620 and WiDr cells with diminished Eps8 further discloses the indispensability of Eps8 in cell locomotion and its participation in tumor progression. Finally, we demonstrate that the expression of Eps8 and FAK is COX-2 dependent and participates in COX-2-induced cell growth. These results were submitted for publication. (d) Collaboration with other sub-projects Still, we know little about how Eps8 causes cancer formation. Our recent studies in v-H-Ras transformed cells indicate that Eps8 is overexpressed and participateing in Raf/MEK/ERK activation since eps8 siRNA significantly knockdown not only Eps8 expression but also Ras-mediated ERK activation. In collaboration with Dr. Liu in the first component of this subproject, we hope to understand how Eps8 promotes ERK activation in v-Ras transformed cells. In addition, how Eps8 expression is regulated, what is the relationship between Eps8 and the other well known cancer-related proteins, such as Stat3, in cancer formation. Recent studies indicate Stat3 is one of the downstream players of v-Src-mediated cell transformation and might be important for human caner formation. In collaboration with Drs. Su and Lai in the third component of this subproject, our preliminary data indicates Stat3 may turn on Eps8 expression since dominant negative Stat3 decrease Eps8 expression in lung cancer PC14PE6/AS2 cell. II-3: Study of the pathogenesis of malignant pleural effusion associated lung adenocarcinoma in Taiwan and Stat3 as a model gene (Wu-Chou Su and Ming-Derg Lai) Lung cancer with its high prevalence, recurrent rate and metastatic potential becomes the leading cause of cancer death in Taiwan. Malignant pleural effusion (MPE) is a poor prognostic sign for patients with non-small cell lung cancer (NSCLC). The generation of MPE is largely regulated by vascular endothelial growth factor (VEGF), and upregulation of VEGF by Stat3 has been observed in several types of 22 tumor cells. Stat3, one of the 7 known STAT (Signal Transducers and Activators of Transcription) family members, is frequently constitutively activated in malignant cells. Activation of Stat3 is involved in regulating many genes such as cell cycle progression, cellular proliferation and survival. In this study, we demonstrate constitutively activated Stat3 in several human lung cancer cell lines and in tumor cells infiltrated in the pleurae of patients with adenocarcinoma cell lung cancers (ADCLC) and MPE. The observations suggest that activated Stat3 plays a role in the pathogenesis of ADCLC. In PC14PE6/AS2 cells, a Stat3-positive human ADCLC cell line, autocrine IL-6 activated Stat3 via JAKs, not via Src kinase. PC14PE6/AS2 cells express higher VEGF mRNA and protein than do Stat3-negative PC14PE6/AS2/dnStat3 cells. In an animal model, PC14P6/AS2/ dnStat3 cells produced no MPE and less lung metastasis than did PC14P6/AS2 cells. PC14PE6/AS2 cells also expressed higher VEGF protein, microvessel density, and vascular permeability in tumors than did PC14P6/AS2/dnStat3 cells. Therefore, we hypothesize that autocrine IL-6 activation of Stat3 in ADCLC may be involved in the formation of malignant pleural effusion by upregulating VEGF. Higher levels of IL-6 and VEGF were also found in the pleural fluids of patients with ADCLC than in patients with congestive heart failure. The autocrine IL-6/Stat3/VEGF signaling pathway may also be activated in patients with ADCLC and MPE (1). Lung cancer cells with constitutively activated Stat3 are more resistant to cisplatin- and paclitaxel-induced apoptosis. Abrogate the Stat3 signaling by EGCG or AG490 sensitized cells with activated Stat3 to chemotherapeutic drugs, but provide survival advantage to cells in which Stat3 was deactivated firstly by transfection with dn-Stat3 (2). Upon treatment with anti-cancer agents, the activation of Stat3 in PC14PE6/AS2 cells was enhanced at earlier period (around 3 hours), and then declined gradually. The further activation of Stat3 in PC14PE6/AS2 cells by paclitaxel is mediated mainly by mitochondria-respiratory-chain-generated ROS, which then leads to PKC- and Jak activation (3). We have also investigated expression of a group of proteins in the pleural effusions from lung adenocarcinom, pneumonia and heart failure by ELISA and identified some proteins with pathological significance. Among them, HER-2/neu (4) and IGF-1 (5) have the greatest potential. We have shown that HER2/neu and IGF-1 may play important roles in the pathogenesis of MPE-associated lung adenocarcinoma and may become ideal tumor markers for pleural effusion diagnosis. In proteomics studies, we have identified many pleural proteins (6, 7). By comparing protein expression patterns of MPE from smoking male and non-smoking female lung cancer patients, we have identified three proteins that are overexpressed in non-smoking females. They are α -1 inhibitor III precursor, apolipoprotein E (APOE), and 23 coatomer protein complex, subunitβ(COPB). APOE has been implicated in tumor invasion. By immunohistochemical studies, APOE was found in the cytoplasm of lung adenocarcinoma cells in patients’ pleurae, suggesting it may contribute to pathogenesis of the disease. Further studies on the significance of overexpression of APOE are undergoing. RNA specimens from cancer cells in MPE were extracted and sent for Affymatrix oligonucleotide microarray analyses. Under non-supervised clustering analyses, female non-smokers and male smokers were subdivided into two groups. We also identify a 29-gene-panel that is able to classify these two groups of patients. We are now expanding the sample size and do better control of sample preparation, and will analyze and present the new data in the near future. Besides the known Stat3 downstream genes, we identify that tissue factor is also upregulated by Stat3 in several lung cancer cell lines. Tissue factor may contribute to tumor cell migration, angiogenesis and increase of vascular permeability. According to the data generated from the current study, Stat3 activation, together with other oncogenic pathways, contributes to the pathogenesis of MPE formation, lung metastases, and drug resistance of lung cancer cells. II-4: The role of hepatitis B virus pre-S mutants in hepatocarcinogenesis (Ih-Jen Su) Although hepatitis B virus (HBV) has been documented to cause hepatocellular carcinoma (HCC), the exact role of HBV in the development of HCC remains enigmatic. Previously, we have identified two types of large HBV surface antigens (LHBs) with deletions at pre-S1 (S1-LHBs) or pre-S2 (S2-LHBs) regions in ground glass hepatocytes (GGH)(Wang et al., 2003). The pre-S mutant LHBs are retained in endoplasmic reticulum (ER) and may escape from immune attack. In HCC patients, the pre-S mutant LHBs could be detected in up to 60% in serum and in HCC tissues, suggesting the potential of pre-S mutant proteins in hepatocarcinogenesis. Therefore, this study is aimed to clarify the role of pre-S mutants during the development of HCC. Here, we have demonstrated that HBV pre-S mutant proteins may contribute to hepatocarcinogenesis via both ER stress-dependent and -independent pathways. (a) ER stress dependent pathway ER stress-dependent pathway is responsible for the calcium release, oxidative stress-associated DNA damages, ER chaperone induction, and COX-2 generation. The HuH-7 cells carrying the pre-S mutant LHBS exhibited enhanced levels of reactive oxygen species (ROS) and oxidative DNA damages. Furthermore, the pre-S mutant LHBs can induce mutations on the X-linked hprt gene. The oxidative DNA damage 24 could also be observed in livers of transgenic mice carrying pre-S mutant LHBs, as well as in GGHs. Therefore, through ER stress signaling pathways, the pre-S mutant LHBs can induce oxidative stress and lead to oxidative DNA damage of HBV-infected hepatocytes (Hsieh et al., 2004). The oxidative DNA damages caused by pre-S mutant LHBs may result in genomic instabilities and mutation of liver cells and ultimately lead to HCC. In addition, we found constitutive or inducible expression of the pre-S mutant LHBs induces the expression of COX-2 and prostaglandin E2 in immortalized mouse liver ML-1 cells or in transformed human HuH-7 cells. Transgenic mice expressing pre-S mutant LHBs expressed higher levels of COX-2 protein in liver and kidney tissues. Similarly, increased expression of COX-2 mRNA was observed in human HCC tissues expressing pre-S mutant LHBs. The COX-2 induction is apparently important for the anchorage-independent growth conferred by the expression of pre-S mutant LHBs (Hung et al., 2004). (b) ER stress independent pathway By complementary DNA microarray analysis, we observed that cyclin A, along with other cell cycle regulated genes, was induced by S2-LHBs. The induction of cyclin A was shown to be initiated via the specific transactivator function of S2-LHBs, independent of ER stress signals (Wang et al., 2005). The expression of △S2-LHBs in hepatocytes led to cell cycle progression under strong ER stress condition and enhanced BrdU-incorporation with multinucleation phenotype. Histopathological examinations revealed that cyclin A expression was enhanced in GGHs, HCC tissues and transgenic mice livers. One interesting finding is the tremendous expression of cyclin A in the cytoplasm of hepatocytes induced by △S2-LHBs. Although cyclin A functions predominantly in the nucleus, cytoplasmic expression of cyclin A may contribute to centrosome duplication. It is therefore interesting to clarify whether the alteration of subcellular localization of cyclin A could affect centrosome duplication that subsequently contributes to cell aneuploidy. Recently, our study suggested that cytoplasmic localization of cyclin A is associated with its N-terminal truncation by a calcium-dependent protease, which is activated by ER stress (Wang et al., 2006). These data suggest that cytoplasmic cyclin A may be initiated by ER stress and may contribute to aberrant centrosome duplication. The increased multinucleation and DNA aneuploidy as observed in S2-LHBs transgenic mice livers supports the potential role of ER stress and impaired cyclin A during the development of HCC. Recently, preliminary studies from our group revealed that △S2-LHBs, but not wild-type or △S1-LHBs, could induce hyper-phosphorylation of tumor suppressor retinoblastoma protein (RB). The △S2-LHBs could directly interact with Jun activation domain-binding protein 1 (JAB1), dissociating JAB1 from the 25 JAB1-IRE1 complex in ER lumen and causing JAB1 to translocate to cell nuclei. JAB1 is an important factor to modulate the level of the cyclin-dependent kinase inhibitor p27Kip1 and targets on the nuclear p27Kip1 to cytosolic 26S proteasome for degradation. The degradation of p27Kip1 will activate the phosphorylation of RB. Therefore, the combined events of oxidative DNA damage and RB hyperphosphorylation may represent two independent events for hepatocellular carcinogenesis associated with the ER viral protein △S2-LHBs (Wang et al., 2006). In conclusion, our studies have showed multiple biological effects of HBV pre-S mutant proteins on hepatocarcinogenesis. Both ER stress-dependent and -independent pathways may act synergistically during the development of HCC. The potential role of HBV pre-S mutants in the pathogenesis of HCC provides us a model for viral carcinogenesis. List of Research Publications: 1) Tzeng-Horng Leu and Ming-Chei Maa. (2002) Tyr-863-phosphorylation enhances focal adhesion kinase autophosphorylation at Tyr-397. Oncogene 21, 6992-7000. 2) Jih I Chuang, Tsuey Y Chang, and Hsiao S Liu. (2003) Glutathione depletioninduced apoptosis of Ha-ras-transformed NIH3T3 cells can be prevented by melatonin. Oncogene 22, 1349-1357. 3) Tsui-Lien Hung, Fen-Fen Chen, Jacqueline M. Liu, Wu-Wei Lai, Ai-Li Hsiao, Wen-Tsung Huang, Helen H.W. Chen, and Wu-Chou Su. (2003) Clinical evaluation of HER-2/neu protein in malignant pleural effusion-associated lung adenocarcinoma and as a tumor marker in pleural effusion diagnosis. Clin. Cancer Res. 9, 2605-2612. 4) Tzeng-Horng Leu and Ming-Chei Maa. (2003) Functional implication of the interaction between EGF receptor and c-Src. Front. Biosci. 8, s28-38. 5) Tzeng-Horng Leu, Shu Li Su, Ya-Chun Chuang, and Ming-Chei Maa. (2003) Direct inhibitory effect of curcumin on Src and focal adhesion kinase activity. Biochem. Pharmacol. 66, 2323-2331. 6) Tsuey-Yu Chang, Wen-Jiuan Tsai, Chao-Kai Chou, Nan-Haw Chow, Tzeng-Horng Leu, and Hsiao-Sheng Liu. (2003) Identifying the factors and signal pathways necessary for anchorage-independent growth of Ha-ras oncogene-transformed NIH/3T3 cells. Life Sci. 73, 1265-1274. 7) Tzeng-Horng Leu, Hsu-Hua Yeh, Ching-Chung Huang, Ya-Chun Chuang, Shu-Li Su, and Ming-Chei Maa. (2004) Participation of p97Eps8 in Src-mediated transformation. J. Biol. Chem. 279, 9875-9881. 8) Jui-Hsiang Hung, Ih-Jen Su, Huan-Yao Lei, Hui-Ching Wang, Wan-Chi Lin, 26 Wen-Tsan Chang, Wenya Huang, Wen-Chang Chang, Yung-Sheng Chang, Ching-Chow Chen, and Ming-Derg Lai. (2004) Endoplasmic reticulum stress stimulates the expression of cyclooxygenase-2 through activation of NF-κB and pp38 mitogen-activated protein kinase. J. Biol. Chem. 279, 46384-46392. 9) Shu-Jem Su, Trai-Ming Yeh, Woei-Jer Chuang, Chung-Liang Ho, Kee-Lung Chang, Hsiao-Ling Cheng, Hsiao-Sheng Liu, Hong-Lin Cheng, Pei-Yin Hsu, and Nan-Haw Chow. (2005) The novel targets for anti-angiogenesis of genistein on human cancer cells. Biochem. Pharmacol. 69, 307-318. 10) Yuan-Chang Dai, Chung-Liang Ho, Yi-Chang Tsai, Yung-Hsiang Hsu, Yu-Chuang Chang, Hsiao-Sheng Liu, Helen Hai-Wen Chen, and Nan-Haw Chow. (2005) Allelic loss of 14q32 in the pathogenesis of gastrointestinal and ampullary malignancies: mapping of the target region to a 17 cM interval. J. Cancer Res. Clin. 131, 94-100. 11) Hui-Ching Wang, Wen-Tsan Chang, Wen-Wei Chang, Han-Chieh Wu, Wenya Huang, Huan-Yao Lei, Ming-Derg Lai, Nelson Fausto, and Ih-Jen Su. (2005) Hepatitis B virus pre-S2 mutant upregulates cyclin A expression and induces nodular proliferation of hepatocytes. Hepatology 41, 761-770. 12) Hong-Lin Cheng, Hsiao-Sheng Liu, Yan-Ju Lin, Helen Hai-Wen Chen, Pei-Yin Hsu, Tsuey-Yu Chang, Chung-Liang Ho, Tzong-Shin Tzai, and Nan-Haw Chow. (2005) Co-expression of RON and MET as a prognostic indicator for patients with transitional cell carcinoma of the bladder. Br. J. Cancer 92, 1906-1914. 13) Wen-Ying Lee, Helen Hai-Wen Chen, Nan-Haw Chow, Wu-Chou Su, Pin-Wen Lin, and How-Ran Guo. (2005) Prognostic significance of co-expression of RON and MET receptors in node-negative breast cancer patients. Clin. Cancer Res. 11, 2222-2228. 14) Jeng-Chang Lee, Ming-Chei Maa, Hsiu-Shan Yu, Jung-Hui Wang, Chia-Kuang Yen, Shan-Tair Wang, Yen-Jen Chen, Yuan Liu, Ying-Tai Jin, and Tzeng-Horng Leu. (2005) Butyrate regulates the expression of c-Src and focal adhesion kinase and inhibits cell invasion of human colon cancer cells. Mol. Carcinog. 43, 207-214. 15) Te-Jung Lu, Chi-Ying F. Huang, Chiun-Jye Yuan, Yuan-Chii Lee, Tzeng-Horng Leu, Wen-Chang Chang, Te-Ling Lu, Wen-Yih Jeng, and Ming-Derg Lai. (2005) Zinc ion acts as a cofactor for serine/threonine kinase MST3 and has a distinct role in autophosphorylation of MST3. J. Inorg. Biochem. 99, 1306-13. 16) Tzeng-Horng Leu, Suparat Charoenfuprasert, Chia-Kuang Yen, Chiung-Wen Fan, and Ming-Chei Maa. (2006) Lipopolysaccharide-induced c-Src expression plays a role in nitric oxide and TNFα secretion in macrophages. Mol. Immunol. 43, 308316. 27 17) Hsuan-Heng Yeh, Wu-Wei Lai, Helen H.W. Chen, Hsiao-Sheng Liu, and Wu-Chou Su. (2006) Autocrine IL-6 induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene (in press) 18) Hui-Ching Wang, Wenya Huang, Ming-Der Lai, and Ih-Jen Su. (2006) Hepatitis B virus pre-S mutants, ER stress, and hepatocarcinogenesis. Cancer Sci. (Invited Review) (in press) 19) Pei-Yin Hsu, Hsiao-Sheng Liu, Chung-Liang Ho, Hong-Lin Cheng, Tzong-Shin Tzai, Tsuey-Yu Chang, How-Ran Guo, and Nan-Haw Chow. (2006) Collaboration of RON and EGFR in human epithelial carcinogenesis. J. Urol. (in revision) 20) Yi-Hsuan Hsieh, Ih-Jen Su, Hui-Ching Wang, Jui-He Tsai, Wen-Wei Chang, Ming-Derg Lai, Huan-Yao Lei, and Wenya Huang. (2006) Hepatitis B virus pre-S2 mutant surface antigen induces retinoblastoma hyperphosphorylation: a new mechanism for HBV-related hepatocarcinogenesis. Gastroenterology (in revision) 21) Chen-Yun Yeh, Nan-Haw Chow, Tsuey-Yu Chang, Hui C. Shen, Jyh-Wei Shin, Jung-Hsien Chiang, S.M. Vincent Tseng, and Hsiao-Sheng Liu. (2006) The importance of Axl and PDGFR- in c-Met-associated bladder carcinogenesis. (manuscript in submission) Sub-project (III) Novel signal transduction mechanisms that mediate anti-apoptotic effects in patho-biology (Principal Investigator: Ming-Jer Tang) Apoptosis and anti-apoptosis have become important issues for modern biomedical science. Mechanisms that trigger apoptosis or anti-apoptosis in cell play very important roles in morphogenesis during development or in patho-physiological conditions, such as carcinogenesis or regeneration of specific organ. Apoptosis signals may come from outside of the cell, work on cell membrane receptor, trigger intracellular death machinery and finally degrade the integrity of cell structure. In this subproject, we have collaboratively made some progress in studying signaling mechanisms regarding to interactions of Fas/Fas-L, substratum rigidity-controlled cell behaviors, such as regulation of integrin activation, FAK phosphorylation, MAPK and cell migration, and counteractions of lithium to ceramide-induced cell death. Dr. B. C. Yang demonstrates that Fas cross-linking could directly activate p38 in T cells in a caspase 8/3-dependent way and p38 MAPK pathway is an auto feed-back switch in Fas signaling that dampens the death event in T cells. Collagen gel via the physical property induced down-regulation of focal adhesion complex proteins in all cells examined, which is mediated by 21 integrin. Dr. M. J. Tang and C. Y. Chou demonstrate that low rigidity of collagen fibrils suppresses activation of 1 integrin as 28 well as FAK397 phosphorylation. On the other hand, low substratum rigidity triggers activation of ERK1/2, which results in augmented cell migration. Finally, Dr. Y. S. Lin works on the novel mechanism by which lithium serves to counteract ceramide-induced apoptosis in immune cells and shows that lithium confers protection from ceramide-induced apoptosis via activation of MEK/ERK/Hsp70 and inhibition of mitochondrial activation. Lithium promotes cell survival by inhibiting PP2A activity and caspase-2 activation. Furthermore, GSK-3 is required in ceramide-induced mitochondrial apoptosis. Roles of lithium in PP2A-mediated Bcl-2 dysfunction and initiator caspases activation have been investigated. Results of our studies should have impact in cancer biology, immunology, and developmental biology. III-1: Fas-triggered non-death signaling pathway in tumor formation and autoimmunity (Bei-Chang Yang) We evaluated the Fas signaling in the presence of tumor/ECM. On one hand, we demonstrated that the cell contact-induced reduction in the Fas-cross linking-induced apoptosis in T cells is tumor cell line-dependant. Along with the suppression of apoptosis, a quick and transient phosphorylation of ERK, p38, and AKT was induced in Jurkat T cells. We further demonstrated that PI3K involved in cell contact-induced suppression of apoptosis. On the other hand, prolonged cell contact reversed the elevated ERK phosphorylation in Jurkat cells in an ERK-specific phosphatasesassociated pathway. Furthermore, prolonged integrin activation by ECM engagement increased cAMP level and activated PKA, which subsequently phosphorylated the Raf at 259 a.a. site leading to inhibition of Raf activity and shut down the MEK/ERK. These findings suggest that anchorage on ECM of tumor can dynamically regulate T cell recruitment. Second, we found that Fas signaling intrinsically activated p38 MAPK pathway by itself to suppress caspase-cascade, which in turn reduced cell death. Inactivation of caspase 8 by Z-IETD eliminated the phosphorylation of p38MAPK in response to Fas treatment. Moreover, phosphor-p38 (pp38) is colocalized with caspase 8. The expression of pp38, reflecting the activity of p38 kinase, was higher in synovial T cells from Rheumatoid arthritis (RA) as compared with that in T cells isolated from normal peripheral blood. Moreover, synovial fluid stimulated the p38 in T cells implicate a role of this kinase in RA. In addition to the novel Fas pathway regulating cell death, we further examined whether caspase-3 may stimulate tumor metastasis. When caspase-3 was introduced into the MCF-7 breast cancer cell line, which was originally caspase-3 deficient, cell motility and lung metastasis were significantly enhanced. In parallel, the caspase-3 expression of A549 lung carcinoma cells was 29 reduced by RNA interference (RNAi) strategy. MCF-7 cells overexpressing caspase-3 did not show significant traits of apoptosis. In addition, the caspase-3 overexpressing MCF-7 cells possessed higher motility and secreted more MMP-2. On the contrary, caspase-3 siRNA reduced motility and invasiveness of A549 cells. By using the in vivo experimental lung metastasis model, we observed that caspase-3 increased the severity of metastasis. Collectively, we demonstrated that caspase 3 might involve in tumor metastasis. III-2: Signaling mechanisms of low rigidity of collagen gel-regulated cell life and death (Ming-Jer Tang and Cheng-Yang Chou) Physical environment has been considered as an important factor in regulating cellular behavior. How mechanical impacts affect cell behaviors is a less studied issue. We have shown that collagen gel, as a soft substratum, down-regulates the level of focal adhesion complex proteins through 21 integrin (1). In this study, we wish to explore cell responses to low rigidity, particularly to examine whether the substratum rigidity of collagen gel affects protein synthesis rates and cell migration, and to delineate the signal transduction mechanisms involved in low rigidity-induced cell responses. When MDCK cells were cultured on collagen gel, phosphorylation sites (407, 577, 861, and 925) of FAK were activated, only FAK397 phosphorylation levels remained low or unaltered. Low rigidity-induced decrease in FAK397 phosphorylation could be observed in various cell lines including fibroblasts and transformed cells. We provide a new concept that FAK397 phosphorylation needs internal force provided from actin filaments, but 1 integrin activation requires preferentially external force from rigid substratum and lipid raft may regulate this process (2). We further showed that low rigidity of collagen gel triggered cell migration in all cell lines examined. Low rigidity-induced cell migration was mediated by a delayed onset of Erk-1/2 phosphorylation which was localized on focal adhesions (3). Collagen gel induced phosphorylation of ERK1/2 within 1 h and the induction could last for more than 8 h. Inhibition of collagen-induced ERK1/2 phosphorylation by MEK inhibitor, UO126, resulted in round up morphology and completely abolished cell migration. Interestingly, the collagen gel-induced ERK1/2 phosphorylation was present in focal adhesions. Moreover, filipin III, a specific inhibitor for caveolae, completely alleviated the collagen gel-induced ERK1/2 phosphorylation. Taken together, low rigidity of collagen fiber induces activation of ERK1/2, which serves to facilitate cell spreading and migration on collagen gel. In addition, low rigidity of collagen gel induced apopotsis in epithelial cells. We found that the low rigidity-induced epithelial cell apoptosis could be mediated by deregulation of AP-1 transcription factor (4). In addition, wild type and dominant negative FAK or DDR1 stably transfected MDCK 30 cells were employed (5, 6). 1. Rigidity of collagen fibrils control collagen gel-induced down-regulation of focal adhesion complex proteins mediated by 21 integrin. (Wang et al, J. Biol. Chem. 278, 21886-21892) We found that focal adhesion complex proteins, including FAK, talin, paxillin and p130, but not vinculin, were decreased within 1 h when MDCK cells were cultured on collagen gel. Collagen gel-induced selective decrease of focal adhesion proteins was observed in all lines of cells examined, including epithelial, fibroblastic and cancer cells. Matrigel also induced selective down-regulation of focal adhesion proteins. However, cells cultured on collagen gel- or matrigel-coated dishes did not show any changes of focal adhesion proteins. These data suggest that the physical nature of the gel, i.e. the rigidity, is involved in the expression of focal adhesion proteins. The collagen gel-induced down-regulation of focal adhesion complex proteins was caused by reduction of protein synthesis and activation of proteases such as calpain and was mediated by α2β1 integrin, but not DDR1. Furthermore, freshly isolated renal proximal tubule (PT) cells, initially exhibited little focal adhesion complex proteins, re-expressed focal adhesion complex proteins in primary cultures when they were cultured on collagen gel- or matrigel-coated dishes. Nevertheless, PT cells cultured on collagen gel or matrigel did not re-express focal adhesion complex proteins in primary culture. Taken together, these data indicate that the substratum rigidity controls expression of focal adhesion complex proteins, which may be of physiological significance. 2. Substratum rigidity of collagen gel controls 1 integrin activation and FAK phosphorylation. (Wei et al, manuscript in preparation) In this study, we wish to explore cell responses to low rigidity particularly to elucidate whether the internal force provided from actin filaments and microtubules affected 1 integrin activation and FAK397 phosphorylation. We examined whether the membrane microdomain provided signal leading to 1 integrin activation by fractionation and MCD, a lipid raft inhibitor. When MDCK cells were cultured on collagen gel, phosphorylation sites (407, 577, 861, and 925) of FAK were activated, only FAK397 phosphorylation levels remained low or unaltered. Low rigidity-induced decrease in FAK397 phosphorylation could be observed in various cell lines including fibroblasts and transformed cells. In addition, 1 integrin activation was down-regulated under low substratum rigidity. Low rigidity-induced down-regulation of 1 integrin activation or FAK397 phosphorlyation was not mediated through FAK or DDR1. Disruption of actin cytoskeleton by cytochalasin D blocked FAK397 31 phosphorylation but not 1 integrin activation, while disruption of microtubules by colcemide had no effect. MCD, a lipid raft inhibitor, inhibited 1 integrin activation in cells cultured on rigid substratum. However, the level of FAK397 phosphorylation remained unaffected. Taken together, our data provides a new concept that FAK397 phosphorylation needs internal force provided from actin filaments, but 1 integrin activation requires preferentially external force from rigid substratum and lipid raft may regulate this process. 3. Mechanical property of collagen fiber-induced cell spreading and migration are mediated by phosphorylation of ERK1/2 localized at focal adhesions (Hsu et al, J. Cell Sci. in review) Previous study demonstrated that cells cultured on collagen gel displayed down-regulation of focal adhesion proteins induced by low rigidity. In this study, we found that cells cultured on collagen gel exhibited higher migration capacity than those cultured on collagen gel-coated dishes, suggesting that low substratum rigidity triggers cell migration. Cells cultured on collagen gel displayed enhanced FAK tyrosine phosphorylation similar with that on collagen gel-coated dishes, with exception that FAK397. FAKY397 phosphorylation was spared. In contrast, low rigidity induced delayed onset but persistent phosphorylation of ERK1/2. Inhibition of collagen gel-induced ERK1/2 phosphorylation by MEK inhibitors and ERK2 kinase mutant resulted in cell round up and reduction of collagen gel-induced cell migration. Interestingly, the collagen gel-induced ERK1/2 phosphorylation was present in focal adhesion site, indicating that ERK1/2 activation leads to cell spreading and migration. Moreover, MCD (Methyl-cyclodextrin), lipid raft/caveolae inhibitors, prevented collagen gel-induced phosphorylation of ERK1/2 as well as cell migration. Overexpression of FAKY397F mutant in MDCK cells exhibited higher level of ERK phosphorylation than the overexpression of wild type FAK. Taken together, our data provides a new concept that low rigidity-induced lipid raft-mediated activation of ERK1/2, which is present in focal adhesion to facilitate cell spreading and migration. 4. Deregulation of AP-1 protein family in collagen gel–induced apoptosis mediated by low substratum rigidity. (Wang et al, J. Biol. Chem., in review) In order to delineate whether low substratum rigidity affected cell life and death, we employed various types of cell cultured on type I collagen gel or collagen gel-coated dish. Here we established that collagen gel, but not collagen gel-coating, induced apoptosis of epithelial (NMuMG, BS-C-1, LLC-PK1, NRK-52E and BAEC) but not mesenchymal (HEK 293 and NIH-3T3) or tumor (HK-2, U-373MG, OC-2, 32 DOK, SSC-25, HSC-3 and Chang Liver) cell lines, indicating that low substratum rigidity may trigger cell apoptosis. To delineate whether rigidity of collagen gel controlled epithelial cell apoptosis, we employed collagen gels harboring different rigidity due to cross-linking or physical disruption of collagen fibrils. Collagen gel prepared from older rat-tail tendon contracted cell morphology as well as apoptosis. On the other hand, a reduction in rigidity by sonication of collagen fibrils augmented collagen gel-induced apoptosis. As assessed by rheometry, the rigidity of collagen gel, ranged from 10 to 120 Pascal, was elevated by age effects and lowered by sonication. Low rigidity-induced apoptosis could neither be prevented by Bcl-2 overexpression, nor preceded by mitochondria release of cytochrome c, indicating that the mitochondrial pathway is not involved in low rigidity-induced apoptosis. Low rigidity triggered activation of JNK within 4 h, but induced rapid down-regulation of c-Jun within 1 h and triggered aberrant persistent expression of c-Fos for at least 24 h. Both reduction of c-Jun expression by sh-RNA and overexpression of c-Fos could induce apoptosis in several epithelial cells. Inhibition of low rigidity-induced JNK activation by SP600125 could prevent aberrant c-Fos expression, but only partially blocked low rigidity-induced apoptosis. Taken together, we conclude that low substratum rigidity induces apoptosis in epithelial cells, which is mediated at least in part by deregulation of AP-1 proteins. 5. The function of Discoidin Domain Receptor 1 in HGF-induced branching tubulogenesis of MDCK cells in collagen gel (Wang et al, J. Cell Physiol. 203: 295-304) Discoidin domain receptor I (DDR1) is a receptor tyrosine kinase and serves as the receptor for collagen in addition to integrins. It has been well established that MDCK cells develop branching tubules in three-dimensional collagen gel in the presence of HGF. MDCK cells normally express DDR1. However, the function of DDR1 in this in vitro model system has not been understood. We established stable-transfected MDCK cells harboring DDR1a, DDR1b or dominant-negative DDR1 and cultured these transfectants in collagen gel with HGF (2 ng/ml) for the studies of branching tubule morphogenesis. Whether DDR1 played roles in cell growth, apoptosis and migration was examined. We found that cells over-expressing DDR1a and DDR1b developed shorter tubules with fewer branches in collagen gel. In contrast, dominant-negative DDR1 over-expressed cells could not form tubule structure, but instead developed mostly cell aggregates with multiple long extended processes. Overexpression of DDR1a and 1b in MDCK cells resulted in reduction of cell growth when cells were cultured on collagen gel-coated dishes or collagen gel. On the other hand, dominant-negative DDR1 enhanced cell death on collagen gel, 33 suggesting that DDR1 is involved in maintenance of cell survival. Moreover, over-expression of DDR1a and DDR1b markedly reduced collagen-induced migration capability, whereas dominant-negative DDR1 enhanced it, suggesting that DDR1a and 1b may serve as a negative regulator for α2β1 integrin during migration on collagen substratum. These results indicate that DDR1 plays important role in regulation of HGF-induced branching tubulogenesis by modulating cell proliferation, survival and cell migration. 6. DDR1/SHP-2 signaling complex inhibits 21 integrin-mediated Stat1/3 activation, cell migration and branching tubulogenesis (Wang et al, Mol. Biol. Cell 2006, in press) Regulation of cell migration is an important step for the development of branching tubule morphogenesis in collagen gel. Since collagen fibrils serve as indispensable scaffold matrix for tubulogenesis, functions of the receptor tyrosine kinase for collagen, namely Discoidin domain receptor 1 (DDR1), deserve to be investigated. We previously showed that DDR1 inhibited cell migration and dampened HGF-induced branching tubulogenesis in collagen gel. However, the signal transduction mechanism whereby DDR1 down-regulates cell migration has not been studied. We showed that DDR1 inhibited collagen-induced tyrosine phosphorylation of Stat1 and Stat3 and cell migration triggered by 21 integrin. In addition, upon collagen stimulation, DDR1 interacted with SHP-2 and upregulated the tyrosine phosphatase activity of SHP-2, which subsequently reduced phosphorylation of Stat1 and Stat3 triggered by collagen activation of 21 integrin. We demonstrated that the SH2-SH2 and PTP domains of SHP-2 were responsible for interaction with DDR1, and that both tyrosine phosphorylation sites 703 and 796 of DDR1 were essential for the binding with SHP-2. Mutation of tyrosine 703 or 796 of DDR1 abolished the ability of DDR1 to inhibit the tyrosine phosphorylation of Stat1 and Stat3 and thereby restored collagen-induced cell migration and the HGF-induced branching tubulogenesis in collagen gel. We report here a novel collagen-signaling pathway showing the cross talk between DDR1 and 21 integrin, through an original association of DDR1 and SHP-2 to negatively regulate 21 integrin-dependent signaling. In summary, SHP-2 is required for the DDR1-induced suppression of Stat1 and Stat3 tyrosine phosphorylation, cell migration, and branching tubulogenesis. III-3: Molecular mechanism of ceramide-induced apoptosis: the anti-apoptotic role of lithium (Yee-Shin Lin) Ceramide, a product of sphingolipid metabolism, is generated in response to various stress stimuli. Ceramide may modulate the biochemical and cellular processes 34 that lead to apoptosis. However, the mechanisms by which ceramide regulates apoptotic events are not fully defined. In this project, our studies have shown that sequential activation of caspase-2 and -8 is essential for ceramide-induced mitochondrial apoptosis (Lin et al., 2004). Bcl-2 rescues ceramide-induced apoptosis through blockage of caspase-2 activation. Protein phosphatase 2A (PP2A)-mediated Bcl-2 dephosphorylation is involved in caspase-2 activation induced by ceramide (Lin et al., 2005). Furthermore, lithium confers protection against ceramide-induced apoptosis by activation of MEK/ERK and inhibition of caspase-2 and -8 (Jan et al., manuscript in preparation). We recently show that lithium blocks ceramide-induced apoptosis via inhibition of PP2A activity. Ceramide-induced PP2A activation involves methylation of PP2A C subunit, which lithium suppresses. Lithium causes dissociation of PP2A B subunit from the PP2A core enzyme, whereas ceramide causes recruitment of the B subunit (Chen et al., 2006). Further study demonstrates a role of glycogen synthase kinase-3 (GSK-3) in ceramide-induced apoptosis, in which GSK-3 acts downstream of PP2A and PI3K/Akt and upstream of caspase-2 and caspase-8. In addition to a direct effect of lithium on GSK-3 as previously known, our study indicates that lithium may cause GSK-3 phosphorylation and inactivation through PI3K/PKB- but not ERK-mediated pathway (Lin et al., submitted). Microarray analysis reveals that ceramide upregulates thioredoxin-interacting protein (TXNIP) expression, whereas lithium downregulates its expression. The involvement of TXNIP in p38 MAPK- and JNK-mediated apoptotic signaling pathways is demonstrated (Chen et al., manuscript in preparation). Interestingly, we have also found the involvement of acid sphingomyelinase and ceramide in spontaneous thymocyte apoptosis and lithium may prevent cell death by inhibiting GSK-3 and caspase-2 activation (Chien et al., manuscript in preparation). Taken together, our studies show both transcription-independent and transcription-dependent pathways of ceramide-induced apoptotic cell death, and lithium confers an anti-apoptotic effect in both pathways. 1. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramide- and etoposide-induced apoptosis (Lin et al., Journal of Biological Chemistry 279:40755-40761, 2004) Recently, caspase-2 was shown to act upstream of mitochondria in stress-induced apoptosis. Activation of caspase-8, a key event in death receptor-mediated apoptosis, has also been demonstrated in death receptor-independent apoptosis. The regulation of these initiator caspases, which trigger the mitochondrial apoptotic pathway, is unclear. Here we report a potential regulatory role of caspase-2 on caspase-8 during ceramide-induced apoptosis. Our results demonstrate the sequential events of initiator 35 caspase-2 and caspase-8 activation, Bid cleavage and translocation, and mitochondrial damage followed by downstream caspase-9 and -3 activation and cell apoptosis after ceramide induction in T cell lines. The expression of tBid and reduction in mitochondrial transmembrane potential were blocked by caspase-2 or caspase-8, but not caspase-3, knockdown using an RNA interference technique. Ceramide-induced caspase-8 activation, mitochondrial damage, and apoptosis were blocked in caspase-2 short interfering RNA-expressing cells. Therefore, caspase-2 acts upstream of caspase-8 during ceramide-induced mitochondrial apoptosis. Similarly, sequential caspase-2 and caspase-8 activation upstream of mitochondria was also observed in etoposide-induced apoptosis. These data suggest sequential initiator caspase-2 and caspase-8 activation in the mitochondrial apoptotic pathway induced by ceramide or etoposide. 2. Bcl-2 rescues ceramide- and etoposide-induced mitochondrial apoptosis through blockage of caspase-2 activation (Lin et al., Journal of Biological Chemistry 280:23758-23765, 2005) Recent studies indicate that caspase-2 is involved in the early stage of apoptosis before mitochondrial damage. Although the activation of caspase-2 has been shown to occur in a large protein complex, the mechanisms of caspase-2 activation remain unclear. Here we report a regulatory role of Bcl-2 on caspase-2 upstream of mitochondria. Stress stimuli, including ceramide and etoposide, caused caspase-2 activation, mitochondrial damage followed by downstream caspase-9 and -3 activation, and cell apoptosis in human lung epithelial cell line A549. When A549 cells were pretreated with the caspase-2 inhibitor benzyloxycarbonyl-Val-Asp(-OMe)-Val-Ala-Asp(-OMe)-fluoromethyl ketone or transfected with caspase-2 short interfering RNA, both ceramide- and etoposide-induced mitochondrial damage and apoptosis were blocked. Overexpression of Bcl-2 prevented ceramide- and etoposide-induced caspase-2 activation and mitochondrial apoptosis. Furthermore, caspase-2 was activated when A549 cells were introduced with Bcl-2 short interfering RNA or were treated with Bcl-2 inhibitor, which provided direct evidence of a negative regulatory effect of Bcl-2 on caspase-2. Cell survival was observed when caspase-2 was inhibited in Bcl-2 silencing cells. Blockage of the mitochondrial permeability transition pore and caspase-9 demonstrated that Bcl-2-modulated caspase-2 activity occurred upstream of mitochondria. Further studies showed that Bcl-2 was dephosphorylated at serine 70 after ceramide and etoposide treatment. A protein phosphatase inhibitor, okadaic acid, rescued Bcl-2 dephosphorylation and blocked caspase-2 activation, mitochondrial damage, and cell death. Taken together, ceramide and etoposide induced 36 mitochondria-mediated apoptosis by initiating caspase-2 activation, which was, at least in part, regulated by Bcl-2. 3. Ceramide in apoptotic signaling and anticancer therapy (Lin et al., Current Medicinal Chemistry, 2006, in press, review article) Ceramide, a product of sphingolipid metabolism, is generated in response to various stress stimuli, such as tumor necrosis factoragents, and irradiation. Ceramide may modulate the biochemical and cellular processes that lead to apoptosis. However, the mechanisms by which ceramide regulates apoptotic events are not fully defined. It is believed that the biological effect of ceramide depends on its concentration, the activation or differentiation status of the cell, and the time frame of action. Here, we discuss the metabolism and cell apoptotic signaling of ceramide. The involvement of protein kinases (i.e. PI3K/Akt and GSK-3) and protein phosphatases (i.e. PP1 and PP2A), Bcl-2 family proteins, mitochondrial damage, and caspase cascade activation are demonstrated. Further, ceramide and its derivatives have recently been incorporated into strategies for anticancer therapies. An understanding of the apoptotic signaling pathways mediated by ceramide may shed light on its potential for therapeutic intervention. 4. Lithium inhibits ceramide- and etoposide-induced PP2A methylation, Bcl-2 dephosphorylation, caspase-2 activation and apoptosis (Chen et al., Molecular Pharmacology, 2006, in press) Lithium confers cell protection against stress and toxic stimuli. Although lithium inhibits a number of enzymes, the anti-apoptotic mechanisms of lithium remain unresolved. Here we report a novel role of lithium on the blockage of ceramide- and etoposide-induced apoptosis via inhibition of protein phosphatase 2A (PP2A) activity. Overexpression of PP2A resulted in caspase-2 activation, mitochondrial damage, and cell apoptosis that were inhibited by okadaic acid (OA) and lithium. Lithium and OA abrogated ceramide- and etoposide-induced Bcl-2 dephosphorylation at serine 70. Furthermore, ceramide- and etoposide-induced PP2A activation involved methylation of PP2A C subunit, which lithium suppressed. Lithium caused dissociation of PP2A B subunit from the PP2A core enzyme, whereas ceramide caused recruitment of the B subunit. Taken together, lithium exhibited an anti-apoptotic effect by inhibiting Bcl-2 dephosphorylation and caspase-2 activation, which involved, at least in part, a mechanism of downregulating PP2A methylation and PP2A activity. 5. Glycogen synthase kinase-3 acts downstream of PP2A and PI3K/PKB and upstream of caspase-2 in ceramide-induced T cell mitochondrial apoptosis 37 (Lin et al., submitted) The signaling of glycogen synthase kinase-3 (GSK-3) has been implicated in stress-induced apoptosis. The pro-apoptotic role of GSK-3 remains unclear, however. Here we show the involvement of GSK-3 in ceramide-induced T cell mitochondrial apoptosis. Ceramide induced GSK-3 activation via protein dephosphorylation at serine 9. We previously reported that ceramide induced caspase-2 and -8 activation, Bid cleavage, mitochondrial damage, and apoptosis. In this study, we found that caspase-2 activation and the subsequent apoptotic events were abolished by the GSK-3 inhibitors lithium chloride and SB216763 and by GSK-3 knockdown using short interfering RNA. We also found that ceramide-activated protein phosphatase 2A (PP2A) indirectly caused GSK-3 activation, and that PP2A-regulated phosphatidylinositol-3-kinase (PI3K)/protein kinase B (PKB) was involved in GSK-3 activation. These results indicate a role for GSK-3 in ceramide-induced apoptosis, in which GSK-3 acts downstream of PP2A and PI3K/PKB and upstream of caspase-2 and caspase-8. 6. Ceramide-mediated thioredoxin-interacting protein expression causes JNK and p38 MAPK activation and apoptosis in T cells (Chen et al., manuscript in preparation) The transcriptional regulation of ceramide on apoptotic signaling remains unclear. Using microarray analysis, we showed in this study that ceramide induced the transcription of thioredoxin-interacting protein (Txnip), an endogenous inhibitor of thioredoxin, in T cells. The increased Txnip mRNA and protein expression was further confirmed by RT-PCR, Western blotting, and confocal microscopy. Co-localization of Txnip and thioredoxin after ceramide treatment could be detected by confocal microscopy. In addition, ceramide inhibited the thioredoxin activity. Because thioredoxin might inhibit apoptosis-regulating signal kinase 1 (ASK1) and downstream p38 MAPK and JNK, we therefore investigated the effect of ceramide on p38 MAPK and JNK activities. First, ceramide caused p38 MAPK and JNK activation time-dependently. Ceramide-induced cell apoptosis was partially inhibited by p38 MAPK inhibitor SB203580 and JNK inhibitor SP600125. Ceramide regulated p38 MAPK and JNK phosphorylation via a transcriptional mechanism which was demonstrated by cyclohexamide treatment. Furthermore, ceramide also activated ASK1 by causing an increase in activating residue Thr-845 phosphorylation and a decrease in PKB-mediated inhibitory residue Ser-83 phosphorylation. Therefore, our results show that ceramide exhibits a transcriptional regulation of Txnip-thioredoxin signaling to activate ASK1, which then trigger p38 MAPK and JNK activation leading to apoptosis. Meanwhile, ceramide may also activate ASK1 through 38 inactivation of PKB. 7. Lithium confers protection from ceramide-induced T cell apoptosis via activation of MEK/ERK (Jan et al., manuscript in preparation) Lithium, long being used for the treatment of mood disorders, was found to protect neuronal cells from apoptosis in recent years. Lithium has also been shown to enhance lymphocyte proliferation, the response of T cells to IL-2, and the production of IL-2. In this study, the effect of lithium on immune cell apoptosis induced by ceramide was investigated. Treatment of 10I T hybridoma cells with ceramide showed apoptotic characteristics that were inhibited by lithium, but not by sodium and potassium. In order to test whether lithium would cause an effect on ceramide-induced mitochondrial activation, our studies showed that ceramide increased the percentages of cells with mitochondrial membrane potential reduction that was inhibited by lithium treatment. Moreover, ceramide-induced caspase-3 activation was abolished by lithium treatment. In addition to 10I T cells, freshly isolated mouse splenocytes also underwent apoptosis when stimulated by ceramide and this effect was blocked by lithium. Further investigation revealed that lithium augmented MEK and ERK phosphorylation. The MEK inhibitor PD98059 reduced lithium-induced MEK/ERK activation and cell survival. Akt phosphorylation was inhibited by ceramide and elevated by lithium, but lithium could not block ceramide-mediated suppression of Akt. Treatment with Raf-1 inhibitor caused reduction in ceramide-induced apoptosis, suggesting that Raf-1 was involved in apoptotic pathway. In search for the downstream target of ERK, studies indicated that lithium enhanced heat shock protein 70 (Hsp70) expression and Hsp inhibitor moderately abolished lithium-mediated protection of ceramide-induced apoptosis. Taken together, these results indicated that lithium stimulated a survival pathway which involved activation of MEK/ERK and Hsp70, and inhibited ceramide-induced mitochondrial dysfunction, caspase-3 activation and apoptosis. 8. Lithium blocks spontaneous thymocyte apoptosis by activating p38 MAPK and inhibiting glycogen synthase kinase-3(Chien et al., manuscript in preparation) Large number of cells which have not successfully completed T-cell receptor rearrangement or have low affinity for self-MHC/peptide are eliminated by apoptosis during thymus development. Spontaneous thymocyte apoptosis (STA) may, at least in part, mimic this process in vitro. STA appeared to be a common process in different strains of mice, including BALB/c, C3H/HeN, and B57BL/6. Lithium has been shown to play an anti-apoptotic role. In this study, the molecular mechanisms of STA 39 and the anti-apoptotic effects of lithium were studied. Mouse thymocytes treated with caspase inhibitors, including caspase-1 (zYVAD-fmk), -2 (zVDVAD-fmk), -3 (zDEVD-fmk), -8 (zIETD-fmk), and pan caspase inhibitor zVAD-fmk, could prevent STA. However, STA was not inhibited by caspase-9 inhibitor zLEHD-fmk and mitochondrial transmembrane potential pore stabilizers, bongkrekic acid and cyclosporin A. In addition, the reduction of mitochondrial transmembrane potential could only be detected after 12 h. Our results suggested the dependence of caspase-2, -8, and -3 activation and the independence of mitochondrial pathway in early stage of STA. We had also observed acid sphingomyelinase (ASM) expression and ceramide generation in STA. Consistent with this result, inhibition of ASM by chlorpromazine hydrochloride reduced STA and caspase-3 activation. Lithium was able to inhibit STA and decrease caspase activity, but did not reduce ASM production. To mimic the GSK-3 inhibition of lithium, specific GSK-3 inhibitor SB415286 was used. Results confirmed the dependence of GSK-3 in STA, and GSK-3 phosphorylation on Ser-9 inhibitory residue was increased by lithium. Further results showed that only p38 MAPK inhibitor SB203580 could suppress lithium-conferred protection from STA, but MEK/ERK, PI3K/Akt, and JNK/SAPK inhibitors had no effect. Also, lithium treatment caused p38 MAPK phosphorylation. Further study indicated that p38 MAPK inhibitor blocked lithium-mediated GSK-3 phosphorylation. Based on these results, we suggest that lithium regulates p38 MAPK-mediated GSK-3 inactivation and caspase cascade activation to prevent the induction of STA. The findings in this study reveal the production of ASM/ceramide and the involvement of caspase activation in STA. In addition, lithium protects cells from death is mediated by a mechanism of p38 MAPK activation and GSK-3 inactivation. List of Research Publications: 1) Wei-Shio Hor, Wei-Lune Huang, Yee-Shin Lin, and Bei-Chang Yang. (2003) Cross-talk between tumor cells and neutrophils through the Fas(APO-1, CD95)/FasL system: human glioma cells enhance cell viability and stimulate cytokine production in neutrophils. J. Leukocyte Biol. 73, 363-368. 2) Yi-Ling Chen, Shun-Hua Chen, Jiu-Yao Wang, and Bei-Chang Yang. (2003) Fas ligand on tumor cells mediates inactivation of neutrophils. J. Immunol. 171, 1183-1191. 3) Bei-Chang Yang, Heng-Kai Lin, Wei-Shio Hor, Jun-Yen Hwang, Yu-Ping Lin, Ming-Yie Liu, and Ying-Jan Wang. (2003) Mediation of enhanced transcription of the IL-10 gene in T cells, upon contact with human glioma cells, by Fas signaling through a protein kinase A-independent pathway. J. Immunol. 171, 3947-3954. 4) Meng-Ru Shen, Cheng-Yang Chou, Keng-Fu Hsu, Yueh-Mei Hsu, Wen-Tai Chiu, 40 Ming-Jer Tang, Seth L. Alper, and J. Clive Ellory. (2003) KCl cotransport is an important modulator of human cervical cancer growth and invasion. J. Biol. Chem. 278, 39941-39950. 5) Soon-Cen Huang, Ming-Jer Tang, Ya-Min Cheng, Keng-Fu Hsu, Chung-Liang Ho, and Cheng-Yang Chou. (2003) Enhanced polyadenosine diphosphate-ribosylation in GnRH agonist-treated uterine leiomyoma. J. Clin. Endocrinol. Metab. 88, 5009-5016. 6) Keng-Fu Hsu, Soon-Cen Huang, Jenn-Ren Hsiao, Ya-Min Cheng, Saprina PH Wang, and Cheng-Yang Chou. (2003) Clinical significance of serum human papillomavirus DNA in cervical carcinoma. Obstet. Gynecol. 102, 1344-1351. 7) Yang-Kao Wang, Yao-Hsien Wang, Chau-Zen Wang, Junne-Ming Sung, Wen-Tai Chiu, Shu-Han Lin, Yung-Hen Chang, and Ming-Jer Tang. (2003) Rigidity of collagen fibrils controls collagen gel-induced down-regulation of focal adhesion complex proteins mediated by α2β1 integrin. J. Biol. Chem. 278, 21886-21892. 8) Chun-I Sze, Meng Su, Subbiah Pugazhenthi, Purevsuren Jambal, Li-Jin Hsu, John Heath, Lori Schultz, and Nan-Shan Chang. (2004) Down-regulation of WW domain-containing oxidoreductase induces Tau phosphorylation in vitro. A potential role in Alzheimer's disease. J. Biol. Chem. 279, 30498-30506. 9) Meng-Ru Shen, Ai-Chien Lin, Yueh-Mei Hsu, Tsui-Jung Chang, Ming-Jer Tang, Seth L. Alper, J. Clive Ellory, and Cheng-Yang Chou. (2004) Insulin-like growth factor 1 stimulates KCl cotransport, which is necessary for invasion and proliferation of cervical cancer and ovarian cancer cells. J. Biol. Chem. 279, 40017-40025. 10) Chiou-Feng Lin, Chia-Ling Chen, Wen-Tsan Chang, Ming-Shiou Jan, Li-Jin Hsu, Ren-Huang Wu, Ming-Jer Tang, Wen-Chang Chang, and Yee-Shin Lin. (2004) Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramide- and etoposide-induced apoptosis. J. Biol. Chem. 279, 40755-40761. 11) Li-Jin Hsu, Lori Schultz, Jeffrey Mattison, Yee-Shin Lin, and Nan-Shan Chang. (2005) Cloning and characterization of a small-size peptide Zfra that regulates the cytotoxic function of tumor necrosis factor by interacting with JNK1. Biochem. Biophys. Res. Commun. 327, 415-423. 12) Chau-Zen Wang, Yuei-Mei Hsu and Ming-Jer Tang. (2005) Function of Discoidin Domain Receptor 1 in HGF-induced branching tubulogenesis of MDCK cells in collagen gel. J. Cell. Physiol. 203, 295-304. 13) Nan-Shan Chang, Lori Schultz, Li-Jin Hsu, Jennifer Lewis, Meng Su, and Chun-I Sze. (2005) 17β-estradiol upregulates and activates WOX1/WWOXv1 and WOX2/WWOXv2 in vitro: potential role in cancerous progression of breast and prostate to a premetastatic state in vivo. Oncogene 24, 714-723. 41 14) Chiou-Feng Lin, Chia-Ling Chen, Wen-Tsan Chang, Ming-Shiou Jan, Li-Jin Hsu, Ren-Huang Wu, Yi-Ting Fang, Ming-Jer Tang, Wen-Chang Chang, and Yee-Shin Lin. (2005) Bcl-2 rescues ceramide- and etoposide-induced mitochondrial apoptosis through blockage on caspase-2 activation. J. Biol. Chem. 280, 23758-23765. 15) Nan-Shan Chang, Joan Doherty, Amy Ensign, Lori Schultz, Li-Jin Hsu, and Qunying Hong. (2005) WOX1 is essential for TNF-, UV light-, staurosporine-, and p53-mediated cell death and its tyrosine 33 phosphorylated form binds and stabilizes serine 46-phosphorylated p53. J. Biol. Chem. 280, 43100-43108. 16) Meng-Ru Shen, Yueh-Mei Hsu, Keng-Fu Hsu, Yih-Fung Chen, Ming-Jer Tang, and Cheng-Yang Chou. (2006) Insulin-like growth factor 1 is a potent stimulator of cervical cancer cell invasiveness and proliferation which is modulated by αv β3 integrin signaling. Carcinogenesis (in press) 17) Chiou-Feng Lin, Chia-Ling Chen, and Yee-Shin Lin. (2006) Ceramide in apoptotic signaling and anticancer therapy. Curr. Med. Chem. (in press, invited review paper) 18) Chau-Zen Wang, Hsiao-Wen Su, Yu-Chih Hsu, Meng-Ru Shen, and Ming-Jer Tang. (2006) A DDR1/SHP-2 signaling complex inhibits 21 integrin-mediated Stat1/3 activation and cell migration. Mol. Biol. Cell (in press) 19) Chia-Ling Chen, Chiou-Feng Lin, Chi-Wu Chiang, Ming-Shiou Jan, and Yee-Shin Lin. (2006) Lithium inhibits ceramide- and etoposide-induced PP2A methylation, Bcl-2 dephosphorylation, caspase-2 activation and apoptosis. Mol. Pharmacol. (in press) 42