2-3 COMBUSTION OF ALKANES
advertisement
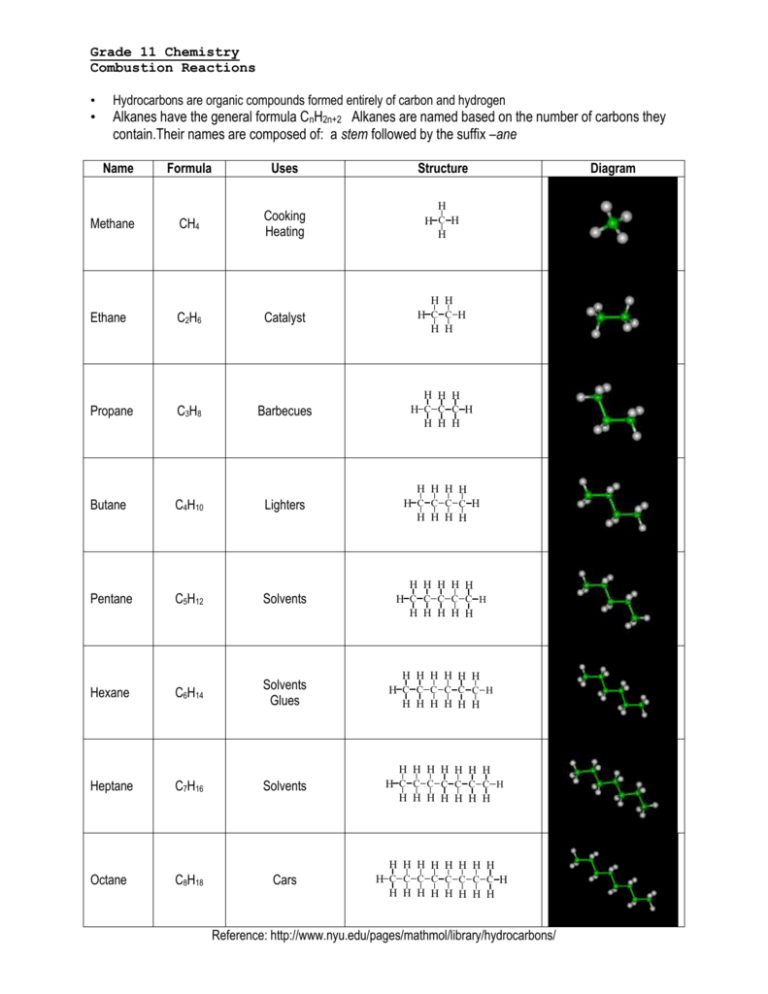
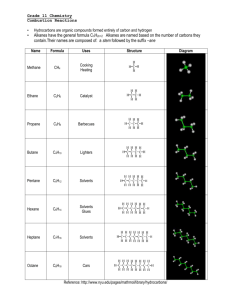
Grade 11 Chemistry Combustion Reactions • • Hydrocarbons are organic compounds formed entirely of carbon and hydrogen Alkanes have the general formula CnH2n+2 Alkanes are named based on the number of carbons they contain.Their names are composed of: a stem followed by the suffix –ane Name Methane Formula Uses Structure CH4 Cooking Heating H H C H H Ethane C2H6 Catalyst H H H C C H H H Propane C3H8 Barbecues H H H H C C C H H H H Butane C4H10 Lighters H H H H H C C C C H H H H H Pentane C5H12 Solvents H H H H H H C C C C C H H H H H H Hexane C6H14 Solvents Glues H H H H H H H C C C C C C H H H H H H H Heptane C7H16 Solvents H H H H H H H H C C C C C C C H H H H H H H H Octane C8H18 Cars H H H H H H H H H C C C C C C C C H H H H H H H H H Reference: http://www.nyu.edu/pages/mathmol/library/hydrocarbons/ Diagram Combustion Of Hydrocarbons A combustion reaction occurs when a________________ burns with __________. Two Types: Complete & Incomplete Combustion A. Complete Occurs when there is excess oxygen Energy ............................. heat, light Hydrocarbons ................... compounds containing hydrogen & carbon Carbon dioxide.................. contributor to the greenhouse effect B. Incomplete Occurs when there is not enough oxygen Carbon monoxide .......... .......poisonous (one, the other or both) Carbon .............................. black soot 1. Write word equations to represent the complete and incomplete combustion of propane, a fuel used in stoves and home heating. 2. a) Write balanced chemical equations for the reactions in #1 above, given that the chemical formula of propane is C3H8. b) How are complete and incomplete combustion different? c) Explain how both complete and incomplete combustion can pose environmental hazards. 3. Why should cars and gas barbeques never be operated in an enclosed space like a garage? Write the balanced chemical reactions for the following: a) Combustion reaction with propane b) Incomplete combustion reaction with butane to produce carbon monoxide and black soot c) Combustion reaction with octane d) Combustion reaction with propane