2 - winterk
advertisement
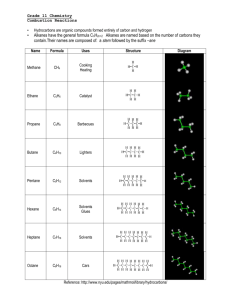
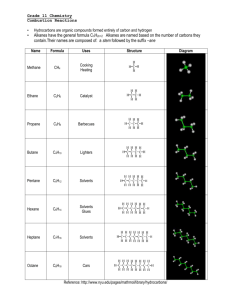
3.1 Hydrocarbons: Types of Reactions Define the following in your own words: a) hydrocarbon d) alkyne b) alkane e) structural isomer c) alkene f) geometric isomer Physical Properties of Hydrocarbons non-polar large hydrocarbons are solids because they have stronger intermolecular bonds, therefore a higher melting & boiling point o ex: coal, plastics smaller hydrocarbons are gases and liquids o ex: methane (natural gas), propane, octane (gasoline), etc. propane (……………..) = low MP/BP octane (……………..) = medium MP/BP coal (……………..) = high MP/BP Combustion Reactions of Hydrocarbons all hydrocarbons burn in the presence of oxygen to produce large amounts of heat and light energy o makes hydrocarbons useful fuels this reaction is an example of a combustion reaction o ALK + O2 CO2 + H2O + called “complete” combustion when lots of oxygen is present and only carbon dioxide & water are produced o ex: complete combustion of propane o C3H8 + O2 CO2 + H2O + your body’s cells undergo complete combustion every moment of every day o you take in O2 through your nose/mouth, your cells split glucose, an organic compound, into useful energy for muscle contraction, digestion, normal body functions (homeostasis) o the CO2 produced by your body’s cells travels through your veins to your lungs where it’s exhaled o the H2O produced is perspiration when not enough oxygen is present, carbon monoxide and carbon soot is produced instead o called “incomplete” combustion o ex: incomplete combustion of propane o C3H8 + O2 CO + H2O + C + many other organic compounds like alcohols, carbohydrates like sugars, etc., undergo combustion as well Ex: Write a balanced chemical equation for the (complete) combustion of pentane. Ex: Write a balanced chemical equation for the (complete) combustion of 1-hexene. HW: p. 188 Practice # 5 Addition Reactions of Alkenes and Alkynes alkenes and alkynes are more reactive than alkanes because of their double/triple bonds double & triple bonds have substantially more energy trapped inside them then single bonds double & triple bonds can be converted to single bonds by performing chemical reactions o energy is released during this process when the double/triple bond is broken, it allows other atoms to combine with the carbon such as hydrogen, halogens or “functional groups” like -OH (hydroxyl) o called an addition reaction o only occurs with alkenes or alkynes because alkanes are already all single bonds addition reaction = when a molecule (hydrogen, halogen) is added to an alkene or alkyne to break apart the double/triple bond in a chemical reaction ex: ethyne + hydrogen gas What can be added to an ALKene/yne in an addition reaction? hydrogen (H2) to saturate the carbons o saturating tastes better foods are examples of natural organic compounds halogens like Br2, Cl2, etc. acids like HCl, HBr, HI, etc. oxygen (forms alcohols, ketones, aldehydes, etc.) nitrogen (forms amines, amides, amino acids) Ex: Use structural diagrams to represent an addition reaction of hydrochloric acid to a propene molecule. Ex: Use structural diagrams to represent an addition reaction of water and 2-butene. when a double/triple bond is present, the molecule is called unsaturated, but if it only has single bonds it is a saturated molecule o ex: saturated vs. unsaturated fats saturated fats are saturated with hydrogen atoms o fats are examples of complex organic molecules polyunsaturated = hydrocarbon containing more than 1 double/triple bond o healthier alternative to saturated fats HW: p. 190 Practice # 6, 7 substitution reaction = when a halogen or hydrogen is substituted for another in an ALK o double/triple bonds aren’t broken but instead hydrogens are replaced with halogens or vice versa o ex: butane vs. 2-fluoro, 3-chloro butane C4H10 vs. C4H8FCl