Specific Heat page 2
advertisement

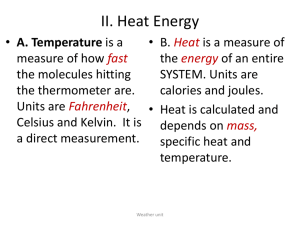
Name: ________________________ Period:______ Date:________________ Unit 1: Energy Specific Heat Ditto Background: Specific Heat, is the measure of the heat energy required to increase the temperature of a unit quantity of a substance by a unit of temperature. For example, the heat energy required to raise water’s temperature 1K (equal to 1 0 C is 4.186 joules per gram. This would be expressed as 4.186 J/(kg·K). The heat energy required to raise air’s temperature 1K (equal to 1 0 C is 1.0 joules per gram. This would be expressed as 1.0 J/(kg·K). It takes less energy to raise the temperature of air than it does water. This is why the temperature of the air changes more rapidly than the temperature of water. More heat energy is required to increase the temperature of a substance with high specific heat than one with low specific heat. You have a table of specific heats of different substances in your ESRT! Part 1 Questions: 1. What is specific heat? ___________________________________________ _____________________________________________________________ 2. Why do you think that large bodies of water have a regulating effect on weather and climate? __________________________________________________ _____________________________________________________________ _____________________________________________________________ 3. What substances temperature would change more rapidly water or Basalt? What is the specific heat of each? ______________________________________ _____________________________________________________________ _____________________________________________________________ 4. Which substance’s temperature would change more rapidly if exposed to the same conditions Granite or Basalt? _______________________________________ _____________________________________________________________ _____________________________________________________________ Part 2: There is also an easy way to understand specific heat…with math and equations! The equation relating heat energy to specific heat capacity, where the unit quantity is in terms of mass is: Q (In Joules) is the heat energy put into or taken out of the substance m (in grams) is the mass of the substance c (in J/(kg·K) is the specific heat capacity ΔT( in 0C or 0K) is the change in temperature. Questions: Let’s test it out! 1. How many Joules does it take to raise 10 grams of water 100C? 2. How many Joules does it take to raise 400 grams of Basalt 400C? 3. How many Joules does it take to raise 40 grams of Dry Air 600C? 4. How many Joules does it take to raise 100 grams of Basalt, Granite, Iron, Copper and Lead 100C? Which one can change temperatures the fastest? 5. A 50 gram piece of iron warms from 0°C to 40°C while absorbing 900 joules. What is the specific heat of iron? 6. A 50 gram sample of an unknown metal warms from 18° to 58°C after absorbing 800 joules. What is the specific heat of the metal?