Specific Heat Practice File

SPECIFIC HEAT
PRACTICE
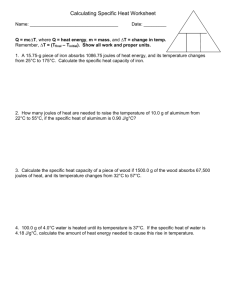
Q = mc ΔT
Problem #1
• How many joules of heat are needed to raise the temperature of 40.0 g of aluminum from 20 °C to 58°C, if the specific heat of aluminum is 0.90 J/g °C?
Problem #2
• When a 140 g sample of aluminum (Al) absorbs 9612 J of energy, its temperature increases from 25 o C to 105 o C.
Find the specific heat of aluminum.
Problem #3
• A 19.75-g piece of iron absorbs
1086.75 joules of heat energy, and its temperature changes from 25 °C to 155°C. Calculate the specific heat capacity of iron
Problem #4
• A certain mass of water was heated with 41,870 Joules, raising its temperature from
21.0 °C to 38.5 °C. Find the mass of the water, in grams.
(Cp of H
2
O = 4.184 J/g °C)
Problem #5
• How many joules of heat are needed to change 60.0 grams of ice at -12.0 °C to steam at
120.0 °C?
• (Cp of H
2
O = 4.184 J/g °C)
Problem #6
• A certain mass of water was heated with 41,870 Joules, raising its temperature from
21.0 °C to 38.5 °C. Find the mass of the water, in grams.
(Cp of H
2
O = 4.184 J/g °C)
Problem #7
• Calculate the number of joules given off when 37.0 grams of steam cools from 120.0 °C to ice at -20.0 °C.
• (Cp of H
2
O = 4.184 J/g °C)
Problem #8
• The specific heat of ethanol is
2.46 J/g o C. Find the heat required to raise the temperature of 173 g of ethanol from 19 o C to 45 o C
Practice #8
• The specific heat of aluminum is 0.88 J/g ⁰ C.
How many joules will it take to make the temperature of a
50 g nugget to go up from 20
⁰ C to 70 ⁰ C?
Practice #9?
• How much heat is needed to warm 0.52 kg of gold from 30 ⁰ C to 120 ⁰ C? (cp = 136
J/kg ⁰ C)
Practice #10
• If it takes 820 Joules of heat to warm a sample of zinc from 0 ⁰ C to 50 ⁰ C, what would be the mass of zinc? (cp= 380 J/kg ⁰ C)
Practice #11
• What is the specific heat of silver if an 80.0 g sample is heated from 24.0
⁰ C to 49.0
⁰ C by adding 468.2J?
Practice #12
• The specific heat of iron is
0.46 J/g ⁰ C. How many joules of heat will it take to make the temperature of a
150 g bar go up from 25 ⁰ C to 60 ⁰ C?
Practice #13
• What is the specific heat of copper if a 75 g sample is heated from
20 ⁰ C to 24 ⁰ C by adding 117 J?
Practice #14
• A 9.5 kg copper sculpture heats up during the day from 24 ⁰ C to 78 ⁰ C. How much energy was absorbed? (cp = 390 J/kg
⁰ C)
Practice #15
• What would be the specific heat of a material that takes 310 joules of energy to warm 40 kg from 50 ⁰ C to 65 ⁰ C?