Energy - cloudfront.net
advertisement

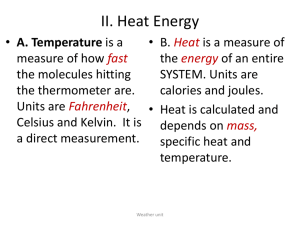
Energy & Chemical Change Chapter 16 16.1 Energy Energy is the ability to do work or produce heat. Energy exists as either potential or kinetic. Physical and chemical changes always involve energy transfer. Energy is measured in joules or calories 1 calorie = 4.184 joules Endothermic reactions absorb energy Exothermic reactions release energy Energy transfer can be calculated by the equation: q = m·ΔT·C q= energy (joules) m = mass (grams) ΔT = change in temperature (Celsius) C = specific heat (Joules/grams·Celsius) What is specific heat? The specific heat of a substance is the heat required to raise the temperature of 1.0 gram by 1.0 °C. Specific heat of water: Gas: 2.02 J/g °C Liquid: 4.20 J/g °C Solid: 2.06 J/g °C Example 1: How many Joules are required to heat 690 grams of nickel from 22 °C to 322 °C? (C = 0.44 J/g °C) Example 2: How many joules are needed to raise the temperature of 20.0 grams of water from 12°C to 25°C? (C = 4.20 J/g °C) Practice Problem 1: Calculate the amount of energy needed to raise the temperature of 20 grams of iron from 10 °C to 40 °C. (C = 0.45 J/g °C) Practice Problem 2: A total of 260 joules of energy were added to a sample of gold. The temperature increased from 10 °C to 20 °C. What is the mass of the sample? (C = 0.13 J/g °C) Energy & Chemical Change Chapter 16 16.2 Heat in Chemical Reactions Thermochemistry is the study of heat changes that accompany chemical reactions and phase changes. The system is a specific part of the universe that contains the reaction being studied. The surroundings is everything else outside the system. System + Surrounding = Universe 16.3 Thermochemical Equations Phase changes require energy. The change from solid to liquid is called the enthalpy (heat) of fusion (ΔHfus). The change from liquid to gas is called the enthalpy (heat) of vaporization (ΔHvap) q = m·ΔH ΔHfus = 334 J/g (s-l) ΔHvap = 2260 J/g (l-g) EX: Calculate the amount of heat required to convert 25.0 grams of ice at -50 °C to vapor at 140 °C. Specific heat of water: Gas: 2.0 J/g °C Liquid: 4.2 J/g °C Solid: 2.1 J/g °C EX: Calculate the amount of heat required to convert 10.0 grams of ice at -5 °C to water at 20 °C. PP: Calculate the amount of heat required to convert 10.0 grams of ice at -20 °C to vapor at 120 °C. PP: Calculate the amount of heat required to convert 50.0 grams of ice at - 20 °C to vapor at 300 °C. Energy & Chemical Change Chapter 16 16.4 Energy Transfer The amount of energy transferred in a reaction can be calculated by the equation: m·(Ti - Tf)·C = m·(Tf - Ti)·C EX: A 25 gram sample of aluminum at 90 °C is dropped into a 100 gram sample of water at 20 °C. What will be the final temperature of the system? (Specific heat of aluminum is 0.90 J/g°C) EX: A sample of copper at 75 °C is dropped into a 50 gram sample of water at 10 °C. The final temperature is 14 °C. What is the initial mass of the metal? (Specific heat of copper is 0.38 J/g °C) PP: A 50 gram sample of aluminum at 80 °C is dropped into a 150 gram sample of water at 10 °C. What will be the final temperature of the system? (Specific heat of aluminum = 0.90 J/g °C) PP: A sample of copper at 99 °C is dropped into a 60 gram sample of water at 15 °C. The final temperature is 16 °C. What is the initial mass of the metal? (Specific heat of copper is 0.38 J/g °C) Energy & Chemical Change Chapter 16 Hess’s Law Hess' Law states that the heat evolved or absorbed in a chemical process is the same whether the process takes place in one or in several steps. EX1: Find the enthalpy for the reaction: CO2(g) + 2H2O(l) => CH4(g) + 2O2(g) using the following data: C(s) + O2(g) => CO2(g) H=-196.7KJ H2O(l) => H2(g) + ½ O2(g) H=142.9KJ CH4(g) => C(s) + 2H2(g) H=37.4KJ EX2: Find th enthalpy for the reaction: 2CO2(g) + 3H2O(l) => C2H6O(l) + 3O2(g) using the following data: 2C2H6O(l) +O2(g) => 2C2H4O(l) + 2H2O(l) 2CO2(g) + 2H2O(g) => C2H4O(l) + 5/2 O2(g) H=-610.5KJ H=1750.5KJ PP1: Find the enthalpy of the reaction: CH4(g) => C(s) + 2H2(g) using the following data: CO2(g) => C(s) + O2(g) H=147.5KJ H2(g) + ½ O2(g) => H2O(l) H=-107.2KJ CO2(g) + 2H2O(l) => CH4(g) + 2O2(g) H=333.9KJ PP2: Find the enthalpy of the reaction: 2HCl(g) + ½ O2(g) => H2O(g) + Cl2(g) Using the following data: CH2Cl2(l) + O2(g) => COCl2(g) + H2O(l) H=-47.5KJ ½ H2(g) + ½ Cl2(g) => HCl(g) H=-230KJ COCl2(g) + 2H2O(l) => CH2Cl2(l) + H2(g) + 3/2O2(g) H=402.5KJ