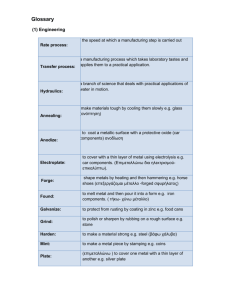
Unit 3 - Section 5.6 2011 Characteristic Physical Property
Grade 9 Academic Science – Unit 2 Chemistry
Characteristic Physical Properties
Section 5.6 Pages 192-197
Characteristic Physical Property
A physical property that is unique to a substance and that can be used to identify the substance
Certain physical properties are unique to each pure substance... and they can be used to identify the pure substance. Unlike chemical tests, characteristics physical properties can be determined
WITHOUT changing the composition of the sample. Thus, the test sample is unchanged.
Three characteristic physical properties are (1) density, (2) freezing / melting point and (3) boiling point.
Density
A measure of how much mass is contained in a given unit volume of a substance.
It is calculated by dividing the mass by volume D = M / V
See Sample Problem 1: Identifying a Metal on Page 193 of Science Perspectives 9
Practice Questions – Using the example “Sample Problem 1” on Page 193, calculate density and, using Table 1, identify the following metals
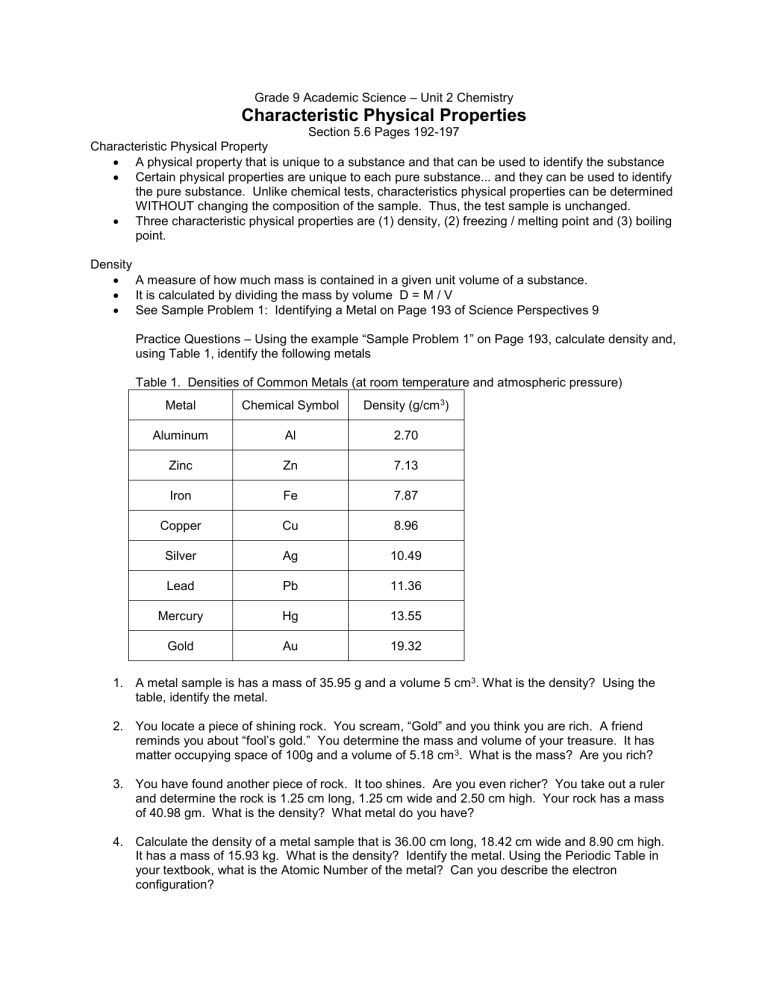
Table 1. Densities of Common Metals (at room temperature and atmospheric pressure)
Metal Chemical Symbol Density (g/cm 3 )
Aluminum
Zinc
Iron
Copper
Al
Zn
Fe
Cu
2.70
7.13
7.87
8.96
Silver
Lead
Ag
Pb
10.49
11.36
Mercury Hg 13.55
Gold Au 19.32
1. A metal sample is has a mass of 35.95 g and a volume 5 cm 3 . What is the density? Using the table, identify the metal.
2. You locate a piece of shining rock. You scream, “Gold” and you think you are rich. A friend reminds you about “fool’s gold.” You determine the mass and volume of your treasure. It has matter occupying space of 100g and a volume of 5.18 cm 3 . What is the mass? Are you rich?
3. You have found another piece of rock. It too shines. Are you even richer? You take out a ruler and determine the rock is 1.25 cm long, 1.25 cm wide and 2.50 cm high. Your rock has a mass of 40.98 gm. What is the density? What metal do you have?
4. Calculate the density of a metal sample that is 36.00 cm long, 18.42 cm wide and 8.90 cm high.
It has a mass of 15.93 kg. What is the density? Identify the metal. Using the Periodic Table in your textbook, what is the Atomic Number of the metal? Can you describe the electron configuration?