Topic1 Finding the purity of marble chips
advertisement

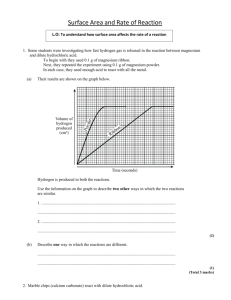
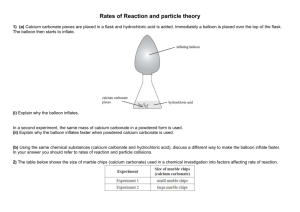
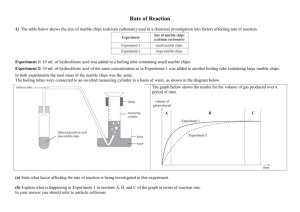
IB Chemistry Topic 1.2 Formulas Practical to find the purity of marble chips In this practical you will be given impure marble chips. Your will be required to find the % purity by finding the volume of gas evolved and from that calculating the mass of calcium carbonate in the marble chips. Practical details. 1. Find the mass accurately of approximately 0.25 g of marble chips. 2. Place in a stoppered flask with a delivery tube connected to a gas syringe. 3. Add 25 cm3 of 4.0 M hydrochloric acid. 4. Find the volume of gas given off by the reaction. 5. Calculate the number of moles of carbon dioxide given off. 6. From this work out the number of moles of calcium carbonate that would give this volume. 7. What mass does this equate to? 8. Therefore what is the percentage of calcium carbonate in the marble chips? Write up Include all practical details and a diagram. Show all steps in the calculation and sources of error. C. M. Wood Page 1 8/03/2016