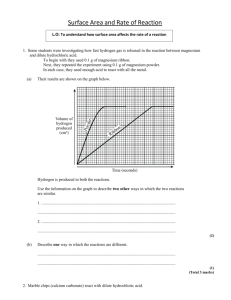
Why study rates of reaction? The rate of a reaction is the speed at which it happens. Some reactions happen very quickly such as sodium reacting with water, whilst other reactions are slower, such as iron rusting. Some reactions take years to occur, such as the chemical weathering of limestone. Some reactions take fractions of a second to happen. Explosions, such as hydrogen burning are very fast reactions. By studying rates we can learn how to control reactions. ● The chemical industry generally requires reactions to happen quickly - more chemicals produced in a quicker time equals more profit! ● Some reactions need slowing down if they are too fast - explosions can be avoided by applying your knowledge of rates of reaction. ● Chemical reactions in food can be controlled to slow spoilage. Various factors can be investigated that affect the rate of a reaction such as: • size of solid particle reacting (surface area) • amount of chemicals reacting (concentration / pressure) • temperature • catalysts • radiation such as light or uv Particles can only react once they have collided. A successful collision will result in reactants changing into products. This idea is known as collision theory. Investigating the effect of particle size on the rate of reaction Marble chips (calcium carbonate) react with hydrochloric acid to produce carbon dioxide gas. calcium carbonate + hydrochloric acid As the gas given off leaves the flask the total mass of the flask and its contents decreases slightly. Readings of the mass loss can be taken. Typically at 30 second to 1 minute intervals. The experiment can be carried out twice, once with large marble chips and again with small marble chips. Plotting a graph of mass loss against time will enable the rate of the reaction to be deduced. calcium chloride + water + carbon dioxide 1. 2. 3. 4. 5. 6. Measure the 50cm3 dilute acid into the conical flask (1 to 2 mol/dm3). Have a loose plug of cotton wool to prevent “spitting” of droplets of liquid. Have the large marble chips of known mass ready. (20g is a suitable amount). Add the marble chips, place the cotton wool in the neck and start the stopwatch. Start taking mass readings immediately. Take readings until the reading on the balance stops changing. Repeat the experiment with small chips. Use this spreadsheet to record your results or draw out a table like the one on the right. Record the mass loss every 30 s for 2.5 minutes and then every minute until the mass stops changing (usually between 10 12 minutes) Time/min Mass loss/g 0 0.00 0.5 1 1.5 Time/m Mass loss/g Mass loss/g in (large chips) (small chips) 0 0.00 0.00 0.5 1 1.5 2 2.5 3 4 5 6 7 8 9 10 If you are not using the spreadsheet then follow these instructions. Plot a line graph with mass loss on the vertical axis and time on the horizontal axis. Plot both sets of result on the same axes. • label axes inc units • title Explaining the effect of increasing particle size • If we grind up a solid to a powder we massively increase the surface area. • We therefore massively increase the rate of any reaction Slow Very fast Explaining the effect of increasing particle size Look at the diagram below. Assume the large cube is a large marble chip with dimensions 6mm x 6mm x 6mm. The second set of cubes are medium sized marble chips, whilst the third set are small marble chips. The volume of each set of cubes is the same (same amount of calcium carbonate) Work out the surface area of each set of cubes. What happens to the surface area as the cubes get smaller? Large marble chip Small marble chips Acid molecule Look at the effect of splitting one large chip into 4 smaller chips. • How does the number of acid molecules in contact with the marble chips change? • How will this affect the number of collisions between the acid and the marble? • How will this affect the rate of reaction? Slower and slower Reactions do not proceed at a steady rate. They start fast and get slower and slower. This is not surprising because the reactant concentration (and the chance of collision) gets lower and lower as time progresses. slower fast 0 25 very slow 75 Percentage completion of reaction stopped 100 Rates and Graphs Shallow gradient Slow reaction Steep gradient Fast reaction Amount of reactant Amount of product • These show the increasing amount of product or the decreasing amount of reactant. Steep gradient Fast reaction Shallow gradient Slow reaction Time Time Rate graphs and reactant concentrations Amount of product All product Mix of reactant And product product Reactant Concentration falls Rate of Reaction falls All reactant Gradient of graph decreases Time 1. Why are the quantities of acid and marble kept the same in both experiments? 2. Explain why the mass decreases as the experiment proceeds. 3. What is the purpose of the cotton wool? 4. How do the results compare for the 2 sizes of marble chips? In your answer, explain how the mass changes over time, comparing each set of results and state what the maximum mass loss is each time. Use your graphs to help answer this question (hint: think about the gradient of the graph). 5. Why do both experiments end with similar mass losses? 6. Why does the mass stop decreasing after a while? 7. What happens to the speed as the reaction proceeds? 8. Which sized chips react faster? 9. Explain your answer to the previous question by using these terms; surface area, area of contact, collisions. 10.Why do explosions sometimes occur in flour mills?