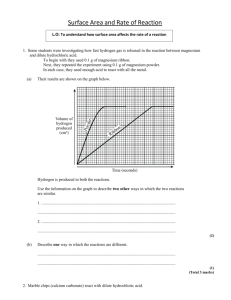
surface area
advertisement

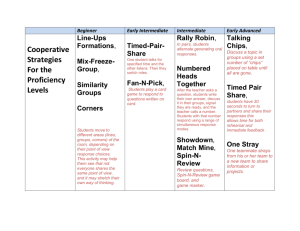
Effect of surface area on reaction rate AIM: To investigate and observe if it is a slower or faster reaction rate when increasing the surface area of the pure calcium carbonate marble chips. HYPOTHESIS: It is predicted that the reaction rate will be much faster when the marble chips have been crushed, increasing its surface area. RISK ASSESSNENT: The hydrochloric acid must not get into the eyes and can irritate skin and deteriorate clothing. To prevent this, must avoid spillages, make sure bottle is closed and wear safety glasses and labcoat. APPARATUS: Marble chips, powdered CaCO3, IM HCI, 2 100ml beakers, stopwatch, 50ml measuring cylinder, electronic scale. METHOD: 1. Measure out 3g of marble chips into beaker. 2. Add 30ml oh HCI solution, start stopwatch. 3. Measure out 3g of powdered CaCO3 into the other beaker. 4. Add 30ml of HCI solution, note time for stopwatch 5. Write down observations of each reaction every 5 minutes. OBSERVATIONS/RESULTS: 3g of Powdered CaCO3: foamed and bubble straight away. Reaction finished in about 40 seconds. 3g of Marble Chips: Slowly bubbling, gradual, reaction still going after 5 min, reaction still continuing after 10 minutes. DISCUSSION: 1. The powdered CaCO3 was faster and the reaction acted instantly in very little time. 2. In terms of the collision theory, since it was powdered there is much more surface area therefore the chances are increased for the atoms to collide. CONCLUSION: It can be concluded that increasing the surface area will speed up the reaction time therefore a faster reaction rate seen from the chemical reaction of carbonate marble chips/powdered reacting with the HCI solution. Regine Imperial 10 White Page 1