Gas laws
advertisement

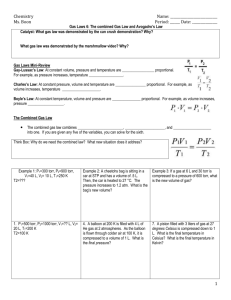
Gas laws 备课时间: 2012 年 11 月 7 日 备课人:丁海林 【学习目标】After completing this chapter, you should be able to: ●describe how the pressure and volume of a gas sample are measured ● list and convert the units of pressure and volume ● explain and complete calculations using Boyle’s law and Charles’ law ● use the absolute temperature scale ●recall standard temperature and pressure conditions and standard laboratory conditions ●recognise the general gas equation and its units ●complete calculations using gas laws and gas equations ● use molar volume at STP and SLC to calculate the volume of a sample of gas ●complete problems using mass–volume and volume–volume stoichiometry. 【课前准备】 ●知识再现 1.000 atm = mmHg = Pa = kPa = bar ●热身练习 The atmospheric pressure at the top of Mount Everest is 253 mmHg. What is the pressure in: a atmospheres? b pascals? c kilopascals? d bars? ●专业术语翻译 1.base peak___________________ 2.beamlines___________________ 3.combined techniques___________________4.fragmentation___________________ 5.free radicals___________________ 6.gas chromatography–mass spectrometry (GC-MS) ___________________ 7.high performance liquid ___________________ 8.chromatography–mass spectrometry (HPLC-MS) ___________________ 9.mass spectrometry (MS) ___________________10.molecular ion___________________ 11.parent molecular ion___________________12.synchrotron___________________ 【构建化学】 ● Boyle’s law:___________________ 【例题解析】 An observation balloon is fi lled with helium gas to a volume of 40 L at a pressure of 1 atm. Calculate the volume when the balloon rises to an altitude where the pressure is 0.2 atm, assuming the temperature remains constant. ● Charles’ law___________________ 1 【例题解析】 A balloon, infl ated outside on a hot day when the temperature is 40°C, has a volume of 5.0 L. What would the volume of the balloon be when it is placed in a cool store at 5°C, assuming the pressure remains constant? ●Amount of gas:___ V=kn 【例题解析】 A 0.10 mol sample of oxygen occupies 2.0 L. What volume would be occupied by 0.25 mol of oxygen? Both samples are at the same temperature and pressure. ●Molar volume of a gas :Vm=V∕n At SLC Vm is L mol−1 At STP is Vm is L mol−1 【例题解析】 1.Calculate the amount of nitrogen gas in a volume of 6.1 L measured at SLC. 2.Determine the volume occupied by 16.0 g of oxygen gas (O2) at SLC. 【巩固提高】 1.Calculate the volume of the following gases at SLC. a 1.4 mol of chlorine (Cl2) b 1.0 × 10−3 mol of hydrogen (H2) c 1.4 g of nitrogen (N2) 2.Calculate the mass of the following gas samples. All volumes are measured at SLC. a 2.8 L of neon (Ne) b 50 L of oxygen (O2) c 140 mL of carbon dioxide (CO2) 2