Flame Test Lab Worksheet: Identifying Metals by Color
advertisement

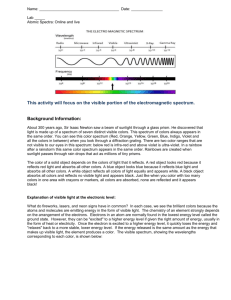
Name: ____________________Date: __________ Period: _______ Pre- Lab: Background Info. / Notes: Every atom consists of a nucleus with tiny electrons whizzing around it. The further away from the nucleus they are, the more energy the electrons have. If a metal is heated, the electrons get enough energy to jump higher away from the nucleus. When they fall back closer to the nucleus, they five off this extra energy as light. Different metals produce different colored light. If we look at the color of the light made when a solution of metal is heated in a flame, we can tell which metal is there. By placing atoms of a metal into a flame, electrons can be induced to absorb energy and jump to an excited energy state, a quantum jump. They then return to their ground state by emitting a photon of light. The amount of energy in the photon determines its color; red for lowest every visible light, increasing through the rainbow until finally violet for the highest energy visible light. Photons outside the visible spectrum may also be emitted, but we can not see them. The arrangement of electrons in an atom determines the size of the quantum jump and thus the energy and colors of the collection of photons emitted, known as the emission spectrum. In this way, the emission spectrum serves as a “fingerprint” of the element to which the atoms belong. We can view the emission spectrum of colors all at once with the naked eye. It will appear to be one color, which we will carefully describe. It is also possible to separate the colors of the emission spectrum by using a spectroscope, which bends light of different energy levels differently. Pre Lab Questions: 1. List the colors of the visible spectrum in order of increasing wavelength. 2. What is meant by the term frequency of a wave? What are units of frequency? Describe the relationship between frequency and wavelength. 3. List the safety precautions associated with using a Bunsen Burner. Name Per: Flame Test Lab Purpose: To determine what colors are characteristic of particular metallic ions in a flame test. To determine the metallic ions in unknowns based on the information gathered in this lab. Materials: Striker Bunsen Burner Known & Unknown Samples Cotton Tipped Applicators **(Write the names of the sample and the numbers of the unknowns taken in the data table)** PART I Procedure: 1. Light the burner. 2. Obtain a small amount of one of the known samples in a small test tube. 3. Dip the cotton tip in the solution & Burn the tip in the hottest part of the flame. 4. Record the color you see (under “naked eye”) when the sample is burnt, being as specific as possible 5. Repeat steps 2 through 4 for all the known & unknown samples. 6. Turn off the burner. Clean up your lab bench. Wash your hands! 7. Determine the identity of the unknowns and put them in the results table. Data Table 1: Flame Tests of Known Solutions Ion in Salt Solution Color of Flame Data Table 2: Tests of Unknown Solutions Unknown Number Color of Flame Results: Unknown Identity of Salt Solution(s) PART II Procedure: 1. Go to the following website http://www.800mainstreet.com/spect/emission-flame-exp.html#Anchortubes. 2. Scroll 4/5 of the way down the page to the Emission Line Spectra for Selected Elements 3. Using the links provided, answer the following questions How do these emission spectra compare in terms of colors and numbers of emission line positions? Are the spectra identical? What if anything is similar? What is different? Examine the spectra for the elements Na, Ne, Hg & He and answer the following questions. FILL IN THE FOLLOWING TABLE WITH YOUR ANSWERS Element with greatest number of visible emission lines Longest wavelength in the spectrum of this atom in nanometers. Color of light for this longest wavelength Post Lab Questions 1. ALL excited electrons give off energy when they return to their ground state from an excited state. Why don’t all heated substances give off colored light? 2. Sodium ions give off a yellow/orange colored flame when heated. Does this mean that all substances that give off a yellow/orange flame when heated contain sodium? Explain your answer. 3. Explain how knowledge of flame tests would be useful to a fireworks designer. 4. What particles are found in the chemicals that may be responsible for the production of colored light? 5. Why do different chemicals emit different colors of light? 6. Why do you think the chemicals have to be heated in the flame first before the colored light is emitted? 7. Colorful light emissions are applicable to everyday life. Where else have you observed colorful light emissions. Are these light emission applications related? Explain.