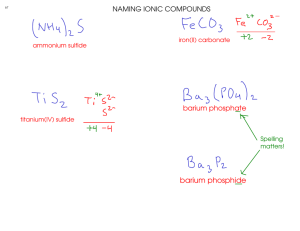
Nomenclature & Mole Application Worksheet
advertisement
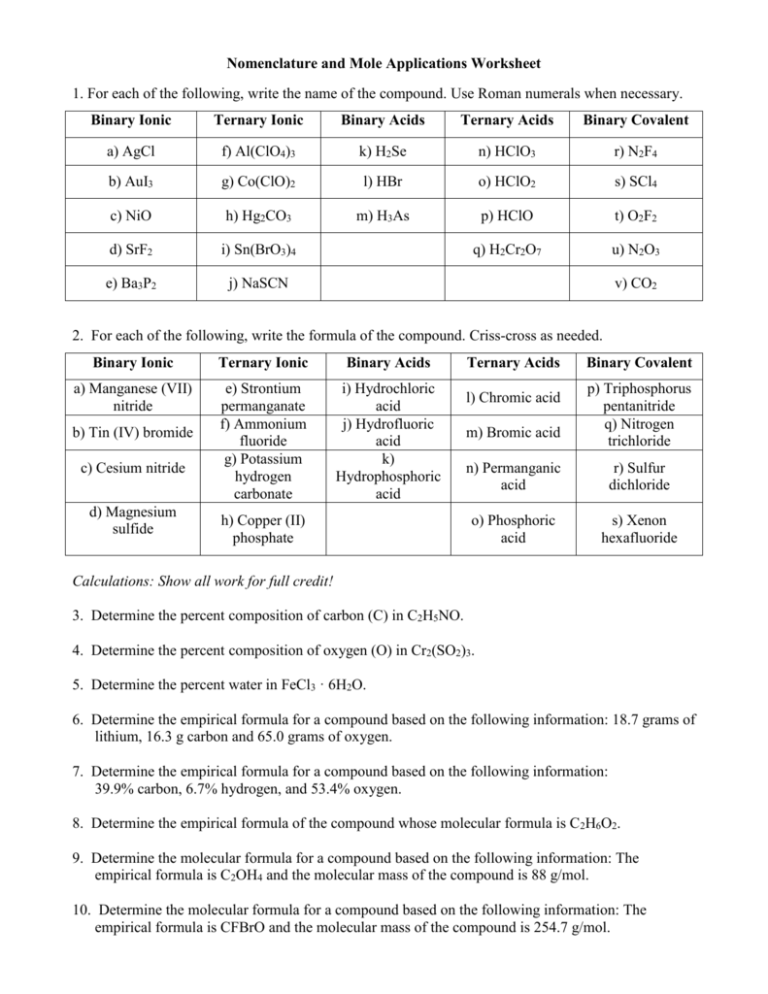
Nomenclature and Mole Applications Worksheet 1. For each of the following, write the name of the compound. Use Roman numerals when necessary. Binary Ionic Ternary Ionic Binary Acids Ternary Acids Binary Covalent a) AgCl f) Al(ClO4)3 k) H2Se n) HClO3 r) N2F4 b) AuI3 g) Co(ClO)2 l) HBr o) HClO2 s) SCl4 c) NiO h) Hg2CO3 m) H3As p) HClO t) O2F2 d) SrF2 i) Sn(BrO3)4 q) H2Cr2O7 u) N2O3 e) Ba3P2 j) NaSCN v) CO2 2. For each of the following, write the formula of the compound. Criss-cross as needed. Binary Ionic Ternary Ionic Binary Acids a) Manganese (VII) nitride e) Strontium permanganate f) Ammonium fluoride g) Potassium hydrogen carbonate i) Hydrochloric acid j) Hydrofluoric acid k) Hydrophosphoric acid b) Tin (IV) bromide c) Cesium nitride d) Magnesium sulfide h) Copper (II) phosphate Ternary Acids l) Chromic acid m) Bromic acid Binary Covalent p) Triphosphorus pentanitride q) Nitrogen trichloride n) Permanganic acid r) Sulfur dichloride o) Phosphoric acid s) Xenon hexafluoride Calculations: Show all work for full credit! 3. Determine the percent composition of carbon (C) in C2H5NO. 4. Determine the percent composition of oxygen (O) in Cr2(SO2)3. 5. Determine the percent water in FeCl3 · 6H2O. 6. Determine the empirical formula for a compound based on the following information: 18.7 grams of lithium, 16.3 g carbon and 65.0 grams of oxygen. 7. Determine the empirical formula for a compound based on the following information: 39.9% carbon, 6.7% hydrogen, and 53.4% oxygen. 8. Determine the empirical formula of the compound whose molecular formula is C2H6O2. 9. Determine the molecular formula for a compound based on the following information: The empirical formula is C2OH4 and the molecular mass of the compound is 88 g/mol. 10. Determine the molecular formula for a compound based on the following information: The empirical formula is CFBrO and the molecular mass of the compound is 254.7 g/mol.