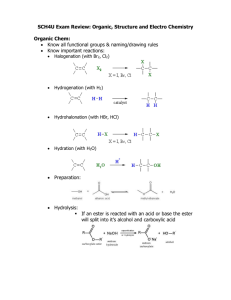
AP Chemistry

A.P. Chemistry
Summer Work 2015
Welcome to Advanced Placement Chemistry. Remember this is a college course we are trying to experience on a high school campus. To prepare for the A.P. test given in May, we need to finish our topics by the end of the third quarter so we can review before the exam. If you have a lab notebook from your chemistry class, keep it, because we will need to review some of the experiments that you may have performed earlier. If you have your labs on notebook paper, placing them in a binder would be sufficient.
Colleges like to see which kinds of labs you have done in an A.P. course.
It is strongly encouraged that prior to leaving this year, you pick up the A.P. Chemistry book from the library ( Chemistry by Zumdahl 7 th edition ) and do the problems and take the notes listed below in the first three chapters in the text. Note which topics you have not learned in your previous chemistry course.
Try to gather any information you can from your text or other notes and try to develop questions to ask as we cover these chapters in class. We will discus these three chapters during the first week of school and will turn in the work the day of the test. I will be available by email before the start of school if you have any questions on the work or in general. My email is mschiller@rusd.k12.ca.us
.
The homework is:
Read and Take notes on Chapter 1. Starting on page 32, do exercises : 25, 28, 30, 34, 45,
51, 63, 69, 76, 83.
Read and Take notes on Chapter 2. Starting on page 70, do exercises : 26, 27, 43, 49, 53,
57, 64, 67, 68, 75.
Read and Take notes on Chapter 3. Starting on page 117, do exercises : 27, 39-49 odd,
61, 67, 73, 86, 92, 103.
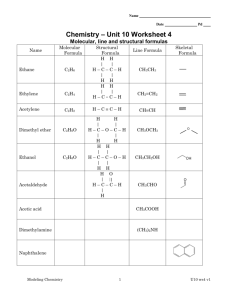
Memorize the polyatomic ion formulas and names on the attached sheet. You will need to know them on the day of our test and throughout the entirety of the course. There are a few memorizing tips given to you in the right-hand column of the list.
Your notes should include any key terms, any examples that you want to include to help your understanding of a concept, any diagrams you feel are necessary, and any key concepts. In your problems, make sure to show any work that you needed to solve the problems and if you don’t know how to solve the problem look in the chapter for examples that might help. Don’t leave any problems blank, at least write down the pertinent information, it might give you a clue how to solve it.
Looking forward to meeting you,
Matt Schiller
Poly Science
Names, Formulas, and Charges of Common Polyatomic Ions
Positive Ions (Cations)
Ammonium NH
4
+ Memorizing Hints:
Mercury (I)
Negative Ions (Anions)
Hg
2
2+ • Anything (except thiocyanate) ending in
"-ite" or "-ate" has Oxygen(s) in formula
Acetate
Carbonate
C
2
H
3
O
2
-
CO
3
2• "-ite" means less Oxygen, "-ate" means more
Bicarbonate/Hydrogen carbonate HCO
3
-
Chlorate ClO
3
• "hypo" means less, "per" (hyper) means more
Chlorite
Hypochlorite
ClO
2
-
ClO • Charges for Sulfur, Chlorine, Phosphorous
Perchlorate
Chromate
Dichromate
Cyanide
Hydroxide
Nitrate
Nitrite
Oxalate
Hydrogen oxalate
Permanganate
Peroxide
Phosphate
Hydrogen phosphate
Dihydrogen phosphate
Phosphite
Sulfate
Bisulfate/ Hydrogen sulfate
Sulfite
Bisulfite/Hydrogen sulfite
Thiocyanate
ClO
4
compounds same as charges of individual ions
CrO
4
2(S 2, SO
4
2, SO
3
2, P 3, PO
4
3, PO
3
3)
Cr
2
O
7
2-
CN -
OH -
NO
3
-
NO
2
-
C
2
O
4
2-
HC
2
O
4
-
MnO
4
-
O
2
2-
PO
4
3-
HPO
4
2-
H
2
PO
4
-
PO
3
3-
SO
4
2-
HSO
4
-
SO
3
2-
HSO
3
-
SCN -
• Adding Hydrogen to beginning of polyatomic lowers negative charge of compound by 1 since
H charge is +1 (SO
4
2, HSO
4
)