Mass-Volume Problems
advertisement

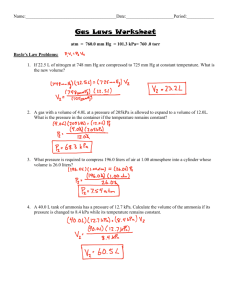
Stoichiometry Worksheet #4: Mass to Volume OHS Chemistry April 23, 2010 1. Given the following equation: MnO2 (s) + 4 HCl (aq) → MnCl2 (aq) + 2 H2O (l) + Cl2 (g) a. How many grams of HCl are required to produce 224. liters of Cl2 at STP? b. How many liters of chlorine (Cl2) at STP will be produced from 1.00 x 103 g of manganese dioxide (MnO2)? 2. Turpentine (C10H16) burns in chlorine (Cl2) to produce carbon (C) and hydrogen chloride (HCl) according to the equation: C10H16 (l) + 8 Cl2 (g) → 10 C (s) + 16 HCl (g) a. How many liters of HCl at STP are produced from 150.0 g of C10H16? b. If 200.0 L of Cl2 at STP reacts with sufficient C10H16, how many grams of C are produced? 3. Given the chemical reaction for the production of table sugar (sucrose): 12 CO2 (g) + 11 H2O (l) → C12H22O11 (s) + 12 O2 (g) a. How many grams of sucrose (C12H22O11) are produced from 224 liters of carbon dioxide (CO2) at STP? b. How many liters of carbon dioxide (CO2) at STP are needed to produce 5.00 pounds of sugar? (1 kg = 2.20 lbs.) c. What mass of water would be needed to combine with 100 liters of CO2 at STP? 4. One of the steps in the production of iron utilizes the following chemical reaction: 3 CO (g) + Fe2O3 (s) → 2 Fe (s) + 3 CO2 (g) a. What mass of Fe2O3 would react with 500.0 liters of CO at STP? b. What volume of carbon dioxide (CO2) at STP is released when 1100 grams of Fe metal is produced by this reaction? c. What volume (in mL) of CO is required to produce 300 mL of CO2 at STP?