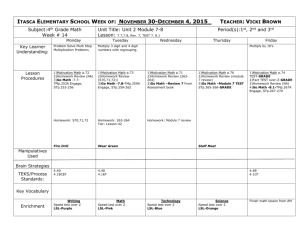
Friday 2/27-- Show all work and FORMULAS ALL WORK MUST BE
advertisement

Friday 2/27-- Show all work and FORMULAS ALL WORK MUST BE ON YOUR PAPER 1. 125 grams of ammonia (NH3) are recovered at STP, how many liters would this equal? 2. A reaction produced 4.25 grams of gas occupying 2.50 liters when collected at STP. What is the density of the gas? Calculate the molar mass of the gas. (2 answers required) 1. A gas collected when the temperature is 35 C and the pressure is 770 mm Hg measures 20.2 mL. Calculate the volume at 25 C and 745 mmHg. 2. A gas occupies a volume of 745 mL at a temperature of 75 C. To what temperature must the gas be lowered if it is to occupy 575 mL? 3. A gas collected at 23 C and 740 mmHg has a volume of 232 mL. What is the volume of the dry gas at STP? 4. A sample of Nitrogen has a volume of 255 mL and a pressure 1.46 atm. If the volume was reduced to 175 mL, what will the new pressure be? 1. 5.10 grams of lithium reacts with Fluorine gas at STP to produce Lithium fluoride in a synthesis reaction. How many liters of Flourine gas is required in the reaction? 2. Using the same balanced reaction: If 625 mL of Flourine gas was used at STP, how many grams of Lithium fluoride could be produced? When copper is mixed with Sulfuric Acid, the products are Copper sulfate, Sulfur dioxide, and water. 3. When copper is mixed with 74.5 grams of Sulfuric Acid, how many mL of the Sulfur dioxide gas is produced if the experiment is conducted at STP? 4. I needed 750 mL of Sulfur dioxide gas to be produced at STP, how many grams of Copper metal would be required? When Iron (III) oxide is mixed with carbon monoxide (g) the products are solid iron and carbon dioxide (g). 5. How many mL of Carbon dioxide can be formed at STP if 625 mL of carbon monoxide is used? 6. How many mL of Carbon dioxide will be produced if 16.0 grams of the Iron (III) oxide is used at STP? 7. If 955 mL of Carbon dioxide gas is collected at 760 mmHg and 0.0 Celsius, how many grams of the Iron (III) oxide were used?