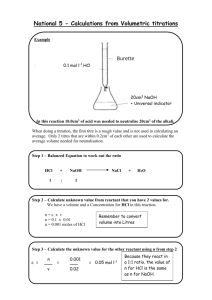
Solubility Answers
advertisement
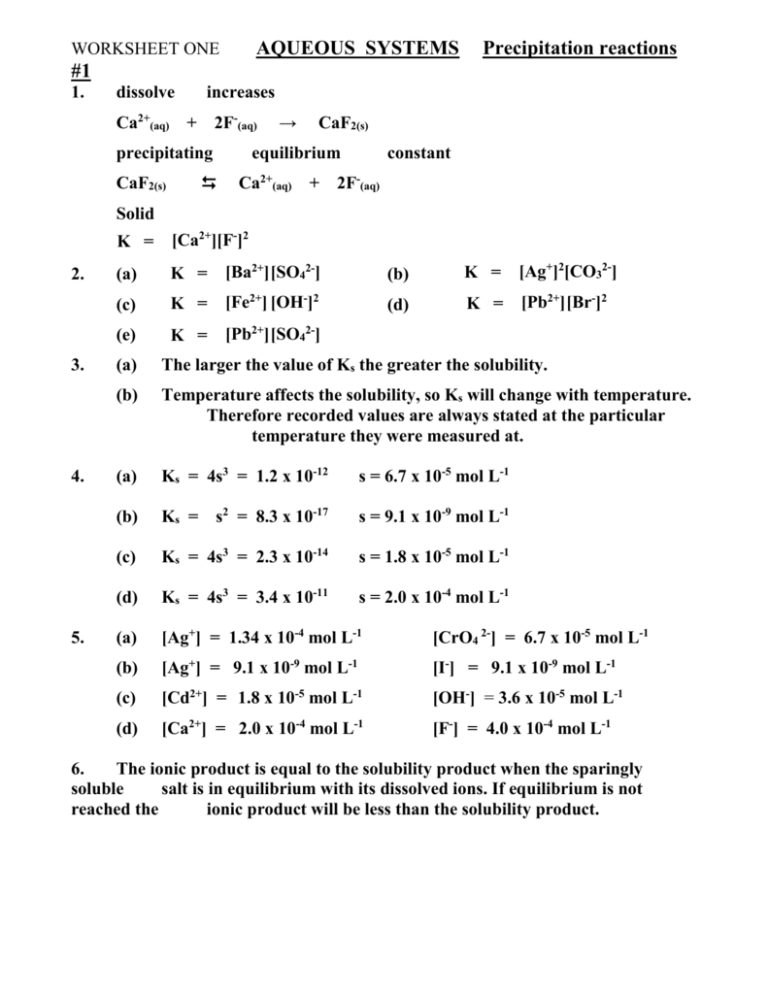
AQUEOUS SYSTEMS WORKSHEET ONE Precipitation reactions #1 1. dissolve increases Ca2+(aq) + 2F-(aq) precipitating CaF2(s) → CaF2(s) equilibrium constant Ca2+(aq) + 2F-(aq) Solid 2. 3. 4. 5. K = [Ca2+][F-]2 (a) K = [Ba2+] [SO42-] (b) K = [Ag+]2[CO32-] (c) K = [Fe2+] [OH-]2 (d) K = [Pb2+] [Br-]2 (e) K = [Pb2+] [SO42-] (a) The larger the value of Ks the greater the solubility. (b) Temperature affects the solubility, so Ks will change with temperature. Therefore recorded values are always stated at the particular temperature they were measured at. (a) Ks = 4s3 = 1.2 x 10-12 s = 6.7 x 10-5 mol L-1 (b) Ks = s2 = 8.3 x 10-17 s = 9.1 x 10-9 mol L-1 (c) Ks = 4s3 = 2.3 x 10-14 s = 1.8 x 10-5 mol L-1 (d) Ks = 4s3 = 3.4 x 10-11 s = 2.0 x 10-4 mol L-1 (a) [Ag+] = 1.34 x 10-4 mol L-1 [CrO4 2-] = 6.7 x 10-5 mol L-1 (b) [Ag+] = 9.1 x 10-9 mol L-1 [I-] = 9.1 x 10-9 mol L-1 (c) [Cd2+] = 1.8 x 10-5 mol L-1 [OH-] = 3.6 x 10-5 mol L-1 (d) [Ca2+] = 2.0 x 10-4 mol L-1 [F-] = 4.0 x 10-4 mol L-1 6. The ionic product is equal to the solubility product when the sparingly soluble salt is in equilibrium with its dissolved ions. If equilibrium is not reached the ionic product will be less than the solubility product. WORKSHEET TWO AQUEOUS SYSTEMS Precipitation reactions #2 0.1 x 0.22 = 4.0 x 10-3 (b) Ks = (1.2 x 10-5)2 = 1.44 x 10-10 1. (a) Ks = 2. (a) (b) The ionic product must have exceeded the solubility product. The ionic product is less than the solubility product. 3. (a) [Ca2+] = 35/200 (6.5 x 10-3) = 1.14 x 10-3 mol L-1 [F-] = 165/200 (4.7 x 10-5) = 3.88 x 10-5 mol L-1 Ionic product = [Ca2+] [F-]2 = 1.72 x 10-12 The ionic product is less than the solubility product so there is no ppt. (b) [Ca2+] = 50/200 (2.2) = 0.55 mol L-1 [CrO42-] = 150/200 (8.7 x 10-1) = 0.653 mol L-1 Ionic product = [Ca2+] [CrO42-] = 3.6 x 10-1 The ionic product is greater than the solubility product so there is a (c) [Zn2+] = 200/350 (2.2 x 10-5) = 1.26 x 10-5 mol L-1 [CO32-] = 150/350 (1.6 x 10-3) = 6.86 x 10-4 mol L-1 Ionic product = [Zn2+] [CO32-] = 8.6 x 10The ionic product is greater than the solubility product so there is a (a) A red-brown ppt of iron III hydroxide formed. Adding FeCl3 crystals caused the [Fe3+(aq) ] to rise and the ion product exceeded the solubility product. Iron III hydroxide precipitated until the ionic product equalled the solubility product again. The is known as the common ion effect. Adding HCl to the equilibrium mixture removed the hydroxide ion as H2O. (H3O+ + OH- → 2H2O). This lowering of the [OH-] caused solid Fe(OH)3 to dissolve as its equilibrium, shown below, moved to the right. Fe(OH)3 (s) Fe3+(aq) + OH-(aq) ppt. ppt. 4. out effect (b) [Sr2+] = 50/70 (8.0 x10-3) = 5.71 x 10-3 mol L-1 [CrO42-] = 20/70 (0.1) = 0.0286 mol L-1 Ionic product = [Sr2+] [CrO42-] = 1.6 x 10-4 The ionic product is greater than the solubility product so there is a 5. ppt. 6. (a) (b) Ks = 4s3 = 8.0 x 10-16 s = 5.9 x 10-6 mol L-1 Ks = 8.0 x 10-16 = [Ca2+] x [OH-]2 [Ca2+] = 8.0 x 10-16 / [OH-]2 = 8.0 x 10-16 / (1 x 10-4)2 Solubility = 8.0 x 10-8 mol L-1