National 5 - Calculations from Volumetric titrations
advertisement
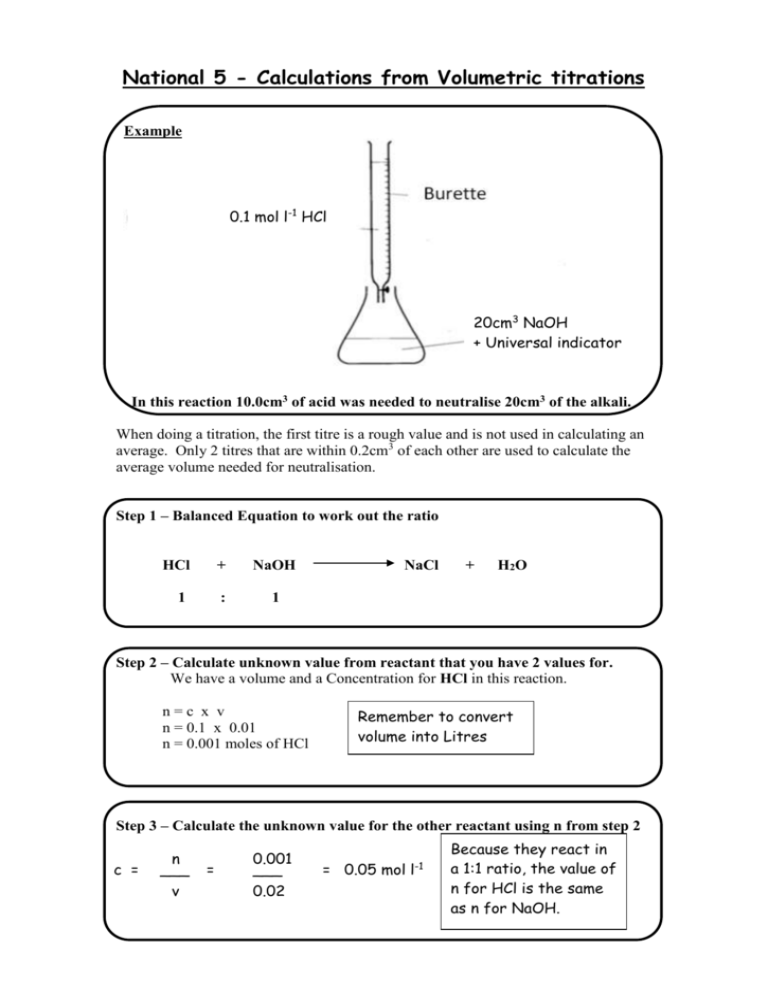
National 5 - Calculations from Volumetric titrations Example 0.1 mol l-1 HCl 20cm3 NaOH + Universal indicator In this reaction 10.0cm3 of acid was needed to neutralise 20cm3 of the alkali. When doing a titration, the first titre is a rough value and is not used in calculating an average. Only 2 titres that are within 0.2cm3 of each other are used to calculate the average volume needed for neutralisation. Step 1 – Balanced Equation to work out the ratio HCl + NaOH 1 : 1 NaCl + H 2O Step 2 – Calculate unknown value from reactant that you have 2 values for. We have a volume and a Concentration for HCl in this reaction. n=c x v n = 0.1 x 0.01 n = 0.001 moles of HCl Remember to convert volume into Litres Step 3 – Calculate the unknown value for the other reactant using n from step 2 c = n ___ v = 0.001 ___ 0.02 = 0.05 mol l-1 Because they react in a 1:1 ratio, the value of n for HCl is the same as n for NaOH. Examples for you to try 1 - What is the concentration of a Sodium hydroxide solution if 20cm3 of the solution is neutralised by A – 20cm3 of 1 mol l-1 Hydrochloric acid B – 40cm3 of 2 mol l-1 Nitric acid C – 40cm3 of 0.25 mol l-1 Sulfuric acid (Careful – not a 1:1 ratio) 2 - What Volume of 0.1 mol l-1 Hydrochloric acid is needed to neutralise A – 15cm3 of 0.02 mol l-1 Potassium hydroxide B – 40cm3 of 0.5 mol l-1 Lithium hydroxide C – 20cm3 of 0.05 mol l-1 Barium hydroxide solution (Careful – not a 1:1 ratio) Answers Question A B C 1 1 mol l-1 4 mol l-1 1 mol l-1 2 0.003 Litres (3cm3) 0.2 Litres (200cm3) 0.02 Litres (20cm3) Alternative method CA x VA Conc Volume of Of Acid x Acid x x nHA Number of H+ ions in formula = CB Conc of = Base x x VB x nOHB Volume Number of OHof Base x ions in formula