A roller coaster ride is a thrilling experience which involves a wealth
advertisement

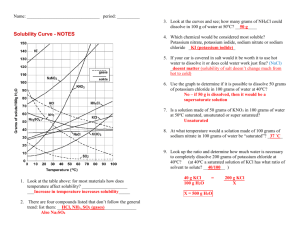
Do Now Name ……………………………………………. Team ………………………. Title: Mass & formula Date ……………………… Which conditions make non polar covalent gas dissolve best a) b) c) d) 1. Low pressure and High Temperature Low pressure and low temperature High pressure and High temperature High pressure and low temperature 9.2g of sodium burns in oxygen. What mass of sodium oxide is made? 4Na + O2 2Na2O 2. 9.8g of sodium is added to water. 2Na + 2H2O 2NaOH + H2 What mass of sodium hydroxide is made? Name a simple covalent gas and draw its Lewis structure Rapid Knowledge Name ……………………………………………. Team ……………………… Title: Oxidation states Date ……………………… Find the oxidatation state of the underlined atom: * Is Na + a cation or an anion What would be the mass of 0.5 moles of NaF How many atoms of fluorine are in the above Solubility graphs Solubility is a measure of how much solute will dissolve in a specific amount of solvent. Usually the solvent is water and the values are based on how much solute will dissolve in 100g of water So let’s just look at the solubility graph On the X axis is temperature of the solution The Y axis shows the number of grams of solute that will dissolve in 100g of water To make this graph less confusing let look at just one substance KCl KCl Example 1 Example 2 If we look at this graph we see that at 0oC, At 80oC. 53 grams of KCl will 29 grams of KCl will dissolve in 100g of water dissolve 100 grams of water It is important that you know how to get this type information from the graph When using a solubility graph it's important to understand how it is created in the first place. solubility of a substance for a given temperature must be determined experimentally This is done by finding out what mass of solute is needed to make a saturated solution in 100 cm3 of water for that temperature(referred to as the solubility). This is repeated for each of the temperatures from 0ºC to 100ºC. The data is then plotted on a temperature/solubility graph,and the points are connected. These connected points are called a solubility curve. Reading Check What Mass of solvent is used as a standard for solubility graphs? What quantity is displayed on the x axis? What is the general trend in solubility for substance shown in this graph? Identify one substance that is an exception? What type of bonding is present in the exception substance that you picked Notes Information Sheet Lecture Notes More explaining Diagrams * Independent practice Definitions quotes and Key points smart phrases Name ……………………………………… Equations Team ………………………. Date ……………………… Title: Solubility graphs 1. Why do the temperatures on the graph only go from 0º C to 100º C ? _____________________________________________________________ _____________________________________________________________ Questions 2. Which substance is most soluble at 60º C ? _____________________________________________________________ 3. Which two substances have the same solubility at 80º C ? _____________________________________________________________ _____________________________________________________________ 4.Which substance’s solubility changes the most from 0º C to 100º C ? _____________________________________________________________ 5.Which substance’s solubility changes the least from 0º C to 100º C ? _____________________________________________________________ 6. What is the solubility of potassium nitrate at 90º C ? _____________________________________________________________ 7. At what temperature does potassium iodide have a solubility of 150 g/ 100 cm3 water ? _______ 8. You have a solution of sodium nitrate containing 140 g at 65º C. Is the solution saturated, unsaturated, or supersaturated ? _____________________________________________________________ 9. You have a solution of potassium chlorate containing 4 g at 65º C. How many additional grams of solute must be added to it, to make the solution saturated ? ________________________________ 10. A solution of potassium iodide at 70º C contains 200 g of dissolved solute in 100 cm3 water. The solution is allowed to cool. At what new temperature would crystals begin to start forming ? _______________________ Exit Ticket Name …………………………………………… Team ………………………. Title:Solubilities curves Date ……………………… * Using the more detailed graph below For questions 1 – 4 an amount of solute is given, and a temperature is stated. If all of the solute could be dissolved in 100 g of water at the given temperature, would the resulting solution be unsaturated, saturated, or supersaturated? 1. 60 g KCl at 70 °C _________ 2. 10 g KClO3 at 60 °C _________ 3. 80 g NaNO3 at 10 °C _________ 4. 70 g CaCl2 at 20 °C _________ For questions 5 – 8 a solute and temperature are given. Tell how many grams of each solute must be added to 100 g of water to form a saturated solution at the given temperature. 5. Pb(NO3)2 at 10 °C _________ 7. NaCl at 20 °C _________ 6. Ce2(SO4)3 at 50 °C _________ 8. K2Cr2O7 at 50 °C _________ For questions 9 and 10 underline the solution that is more concentrated. 9. At 10 °C: a saturated solution of KNO3 or a saturated solution of CaCl2. 10. At 50 °C: a saturated solution of KNO3 or an unsaturated solution of NaNO3 consisting of 90 g of the solute dissolved in 100 g of water. For questions 11 – 12, show your work and circle your final answer. 11. If 115 g KNO3 are added to 100 g of water at 35 °C, how many grams do not dissolve? 12. What mass of KCl would be needed to form a saturated solution if the KCl was dissolved in 200 g of water at 80 °C? HWK * Name ………………………………………… Team ………………………. Title:Solubility Graphs Date ……………………… Refer to the graph below to answer homework questions Solubility Curves of Pure Substances 150 140 130 KI Which of the salts shown on the graph is the least soluble in water at 10oC? 1. Which of the salts shown on the graph has the greatest increase in solubility as the temperature increases from 30 degrees to 60 degrees? 2. Which of the salts has its solubility affected the least by a change in temperature? 3. At 20oC, a saturated solution of sodium nitrate contains 100 grams of solute in 100 ml of water. How many grams of sodium chlorate must be added to saturate the solution at 50oC? 4. At what temperature do saturated solutions of potassium nitrate and sodium nitrate contain the same weight of solute per 100 mL of water? 5. What two salts have the same degree of solubility at approximately 19oC? 6. How many grams of potassium chlorate must be added to 1 liter of water to produce a saturated solution at 50oC? 7. A saturated solution of potassium nitrate is prepared at 60oC using 100.mL of water. How many grams of solute will precipitate out of solution if the temperature is suddenly cooled to 30oC? 8. What is the average rate of increase for the solubility of KNO3 in grams per 100 mL per degree Celsius in the temperature range of 60oC to 70oC? 9. If 50. mL of water that is saturated with KClO3 at 25oC is slowly evaporated to dryness, how many grams of the dry salt would be recovered? 10. Thirty grams of KCl are dissolved in 100 mL of water at 45oC. How many additional grams of KCl are needed to make the solution saturated at 80oC? 11. What is the smallest volume of water, in mL, required to completely dissolve 39 grams of KNO3 at 10oC? 12. What is the lowest temperature at which 30. grams of KCl can be dissolved in 100 mL of water? 13. Are the following solutions saturated, unsaturated or supersaturated (assume that all three could form supersaturated solutions) a. 40. g of KCl in 100 mL of water at 80oC b. 120. g of KNO3 in 100 mL of water at 60oC c. 80. g of NaNO3 in 100 mL of water at 10oC