New 21st Century Chemistry
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
In-text activities
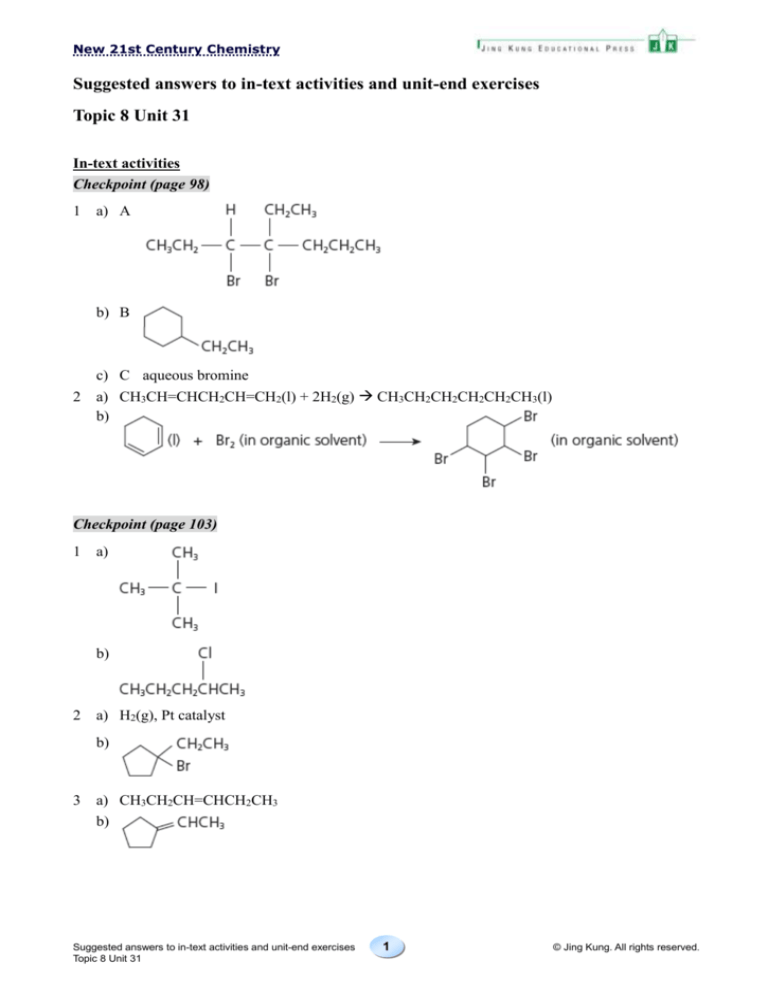
Checkpoint (page 98)
1
a) A
b) B
2
c) C aqueous bromine
a) CH3CH=CHCH2CH=CH2(l) + 2H2(g) CH3CH2CH2CH2CH2CH3(l)
b)
Checkpoint (page 103)
1
a)
b)
2
a) H2(g), Pt catalyst
b)
3
a) CH3CH2CH=CHCH2CH3
b)
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
1
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Checkpoint (page 107)
a)
b)
c)
Checkpoint (page 113)
1
a)
2-bromopentane
b)
iodocyclohexane
c) CH3CH2CH2Cl 1-chloropropane
2
a) Any one of the following:
• Reflux with NaBr + conc. H2SO4
• Reflux with red P + Br2
b) Any one of the following:
• Reflux with NaI + conc. H3PO4
• Reflux with red P + I2
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
2
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Checkpoint (page 118)
1
2
a)
b) X and Y:
Z:
c) X and Y are geometrical isomers.
Checkpoint (page 127)
1 A CH3CH2CH=CH2
B CH3CH2CHBrCH3
major product
C CH3CH2CH2CH2Br
D CH3CH2CH2COOH
2
a)
b)
3
a) The HI(g) formed will be oxidized by concentrated sulphuric acid to iodine.
H2SO4(l) + NaI(s) NaHSO4(s) + HI(g)
8HI(g) + H2SO4(l) 4H2O(l) + H2S(g) + 4I2(s)
Appropriate method — reflux with a mixture of sodium iodide and concentrated phosphoric
acid / reflux with a mixture of red phosphorus and iodine.
b) When heated under reflux, the CH3CHO formed will be oxidized to CH3CH2COOH.
Appropriate method — warm a mixture of excess CH3CH2CH2OH with acidified
Na2Cr2O7(aq), and collect the product by simple distillation.
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
3
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Internet Search & Presentation (page 128)
Introduction
Alcohol intoxication is legally defined by the blood alcohol concentration (BAC) level. However,
taking a blood sample in the field for later analysis in the laboratory is not practical or efficient for
detaining drivers suspected of drink driving. Urine tests for alcohol proved to be just as impractical
in the field as blood sampling.
In the 1940s, breath alcohol testing devices were first developed for use by police. In 1954, Dr.
Robert Borkenstein of the Indiana State Police invented the Breathalyzer, one type of breath alcohol
testing device used by law enforcement agencies today.
How does ethanol get to the lungs?
When a person has taken an alcoholic drink, ethanol passes quickly through the walls of the
stomach and intestines into the bloodstream. Ethanol travels through the bloodstream and reaches
the lung capillaries, which surround the alveoli. As ethanol diffuses into the alveoli, it vaporizes to a
gas.
The concentration of the ethanol in the alveolar air is related to the concentration of the ethanol in
the blood. As the ethanol in the alveolar air is exhaled, it can be detected by the breath alcohol
testing device.
The working principle of a breathalyzer
When orange dichromate ions (Cr2O72–) are brought into contact with ethanol, a redox reaction will
occur and green chromium(III) ions (Cr3+) will form. This colour change is the basis of a
breathalyzer.
The figure below shows the simplified diagram of a breathalyzer. It has three main parts:
• a system to sample the breath of the suspect;
• two small glass bottles containing the reaction mixture (the test bottle and the reference bottle);
• a system of photocells connected to a meter to measure the colour change associated with the
chemical reaction.
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
4
© Jing Kung. All rights reserved.
New 21st Century Chemistry
To measure the breath alcohol content, a suspect breathes into the device. The breath sample is
bubbled through a mixture of potassium dichromate, sulphuric acid and water in the test bottle. The
following chemical reaction takes place:
2Cr2O72–(aq) + 3CH3CH2OH(aq) + 16H+(aq) 4Cr3+(aq) + 3CH3COOH(aq) + 11H2O(l)
To determine the alcohol content of the exhaled air, the device compares identical light beams
which are shone through the reacted mixture and the unreacted mixture in the reference bottle. An
electric current develops if their intensities are different. This causes the needle of the nullmeter to
move from its resting place. The operator then rotates the balance wheel to balance the transmitted
light so as to bring the needle of the nullmeter back to the resting place. This also causes a pointer
on a scale to move and indicate the blood alcohol content of the person being tested.
The ethanol concentration in the expired alveolar air is directly proportional to the ethanol
concentration in the blood. The ratio of the concentration of ethanol in blood to that of the expired
alveolar air is 2 100 : 1.
References
http://www.howstuffworks.com/breathalyzer3.htm
http://rise.duke.edu/apep/teacher/content/pdf/module4.pdf
Checkpoint (page 132)
1 B CH3CH2CH2OH
C CH3CH2COOH
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
5
© Jing Kung. All rights reserved.
New 21st Century Chemistry
2
X
Y
3
a) A
b) B
c) C
Checkpoint (page 139)
1
a)
b)
2
a) i) Concentrated sulphuric acid
ii) To prevent the loss of reactants / products by evaporation.
b)
c) As a flavouring / in perfume
3
a)
b)
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
6
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Checkpoint (page 145)
1
a) A
B
b) C
c) D
2
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
7
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Unit-end exercises (pages 152 – 166)
Answers for the HKCEE (Paper 1) and HKALE questions are not provided.
1
2
a) Any one of the following:
b)
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
8
© Jing Kung. All rights reserved.
New 21st Century Chemistry
c)
d)
e)
3
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
9
© Jing Kung. All rights reserved.
New 21st Century Chemistry
4
5
a)
Structural
type
Structural formula of the isomer
Structural formula for the
compound (if any) formed by
complete oxidation of the alcohol
Primary
Secondary
—
Tertiary
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
10
© Jing Kung. All rights reserved.
New 21st Century Chemistry
b) i) 1-iodopropane
ii) Heat propan-1-ol under reflux with a mixture of red phosphorus and iodine.
iii) PI3
6
7
8
D
C Option C —
B Option B —
9
B
The compound is a tertiary alcohol. It resists oxidation.
Step 1 involves the reduction of an aldehyde to an alcohol.
10 C (1)
The systematic name of the compound is methyl 3-hydroxybutanoate.
(2)
(3) The compound can be oxidized by acidified potassium dichromate solution, thus turning
the dichromate solution from orange to green.
11 a)
b)
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
11
© Jing Kung. All rights reserved.
New 21st Century Chemistry
c)
d)
e)
f)
g)
h)
12 —
13 a)
b) i) Reflux with NaBr and conc. H2SO4; or reflux with red P + Br2
ii) Substitution reaction
c) 1-fluorobutane reacts slower because C–F bond is stronger than C–Br bond.
14 a)
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
12
© Jing Kung. All rights reserved.
New 21st Century Chemistry
b) i) Ethanal is more volatile than ethanol. It can be distilled off. Thus partial oxidation of
ethanol occurs.
ii) Volatile components cannot escape. Thus complete oxidation of ethanol can be achieved.
c) CH3CH2OH + 2[O] CH3COOH + H2O
15 —
16 a) The carbon atom joined to the hydroxyl group is attached to two other alkyl groups / only
one hydrogen atom.
b) i) But-1-ene
ii) Aluminium oxide
iii) Dehydration / elimination
iv)
c)
d) Keep away from naked flames. / Use in a well-ventilated area.
17 a) A — pear-shaped flask / distillation flask
B — condenser
C — anti-bumping granules
b) No stopper at the top of the flask
c)
d)
e)
f)
No jacket on the condenser
Water direction wrong way round
Concentrated sulphuric acid
Oxidation
Hydrogen atoms are lost when ethanol is converted to ethanal.
i) Cr2O72–
ii) From orange to green
Ethanoic acid
CH3COOH
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
13
© Jing Kung. All rights reserved.
New 21st Century Chemistry
18 a) i)
ii) C9H15CHO + H2O
b) i) Esters have fruity smells.
ii) Heat with concentrated sulphuric acid under reflux.
iii) CH3COOH + C9H15CH2OH CH3COOCH2C9H15 + H2O
19 a) i) Hydroxyl group
ii)
iii) Butanoic acid / methylpropanoic acid
b) CH3CH2CH2Br and CH3CHBrCH3
CH3CHBrCH3 is the major product.
20 a) i)
ii) Hydrolysis
b) i) Air condenser
c)
d)
e)
f)
ii) Serving as a fractionating column for separating methanol from the products formed.
Methanol
To ensure even boiling.
B
Any one of the following:
21 a) Warm with acidified potassium dichromate solution.
will change the dichromate solution from orange to green.
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
14
© Jing Kung. All rights reserved.
New 21st Century Chemistry
There is no observable change for
.
b) Mix with aqueous bromine.
CH2=CHCH2OH will change the solution from yellow-brown to colourless.
There is no observable change for
.
c) Mix with sodium carbonate solution.
Effervescence occurs for CH3CH2COOH.
There is no observable change for CH3COCH2OH.
22 a) i) carbon-carbon double bond
ester functional group
ii)
iii) C12H14O2
b) Stereoisomerism — in which atoms are linked in the same way but have different spatial
arrangements.
The structure of compound B is shown below:
In compound A, –CH3 groups are on opposite sides of the C=C bond while in compound B,
both –CH3 groups are on the same side of the C=C bond.
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
15
© Jing Kung. All rights reserved.
New 21st Century Chemistry
c)
23 —
24 a) CH2=CHCH2CH2CHO
b) i)
ii) According to Markovnikov’s rule,
is likely to be in a
greater yield.
OR
i)
ii) According to Markovnikov’s rule,
is likely to be in a greater
yield.
25 —
26 Put three solutions (each containing 2 cm3 of ethanol and 1 cm3 of dilute silver nitrate solution)
into different test tubes.
Place the test tubes in a water bath at 60 °C.
Add 5 drops of chloroethane, bromoethane and iodoethane separately to the test tubes.
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
16
© Jing Kung. All rights reserved.
New 21st Century Chemistry
A general equation for the hydrolysis is:
CH3CH2 — X + H2O CH3CH2 — OH + H+ + X–
The silver nitrate solution is used to follow the rate of hydrolysis of the haloalkanes. Halide ions
produced from the hydrolysis would form a precipitate with the silver ions.
Ag+(aq) + X–(aq) AgX(s)
The rate of hydrolysis of haloalkanes is related to how easily the C–X bond breaks. As the C–I
bond is weaker than the C–Br and C–Cl bonds, the C–I bond breaks most readily. Hence the
rate of hydrolysis of iodoethane is the highest. The precipitate appears first in the test tube
containing it.
As the C–Cl bond is the strongest, the bond is the most difficult to break. Hence the rate of
hydrolysis of chloroethane is the lowest.
Suggested answers to in-text activities and unit-end exercises
Topic 8 Unit 31
17
© Jing Kung. All rights reserved.