New 21st Century Chemistry
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
In-text activities
Discussion (page 40)
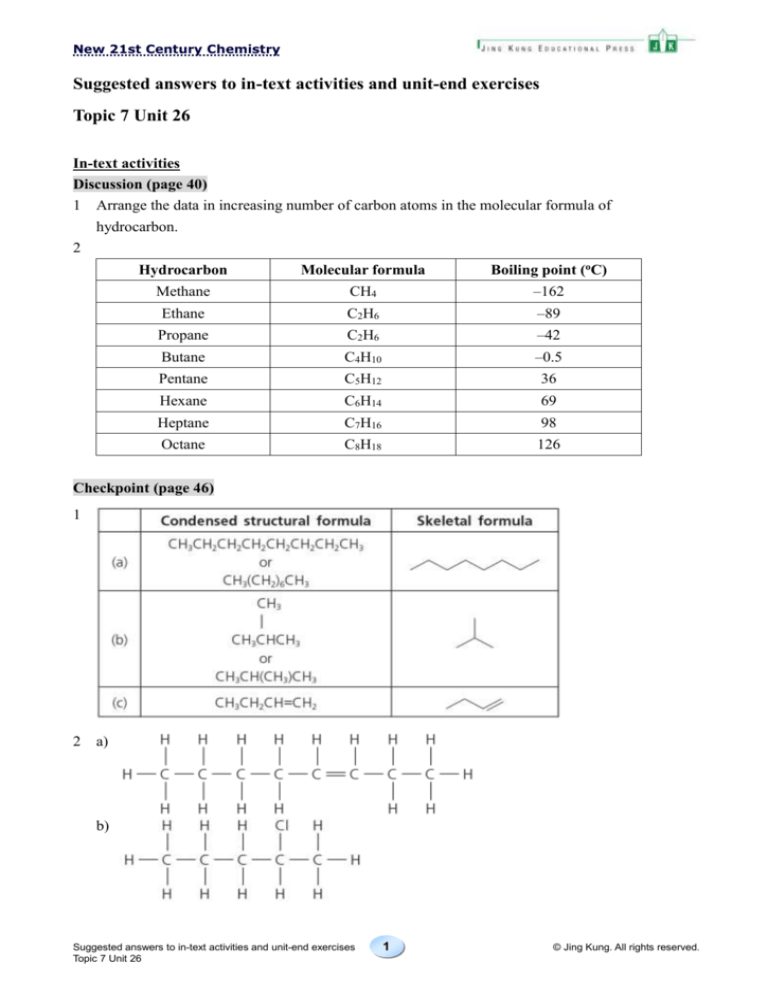
1 Arrange the data in increasing number of carbon atoms in the molecular formula of
hydrocarbon.
2
Hydrocarbon
Molecular formula
Boiling point (oC)
Methane
CH4
–162
Ethane
C2H6
–89
Propane
C2H6
–42
Butane
C4H10
–0.5
Pentane
C5H12
36
Hexane
C6H14
69
Heptane
C7H16
98
Octane
C8H18
126
Checkpoint (page 46)
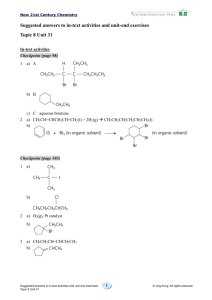
1
2
a)
b)
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
1
© Jing Kung. All rights reserved.
New 21st Century Chemistry
c)
Checkpoint (page 47)
a) C7H16
b) Condensed structural formula:
Skeletal formula:
or
Checkpoint (page 57)
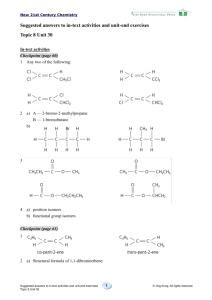
1 a)
methylpropane
b)
3-methylpentane
c)
3,4,4-trimethylheptane
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
2
© Jing Kung. All rights reserved.
New 21st Century Chemistry
d)
4-ethyl-2,4-dimethylhexane
e)
4-methyloctane
2
a)
b)
3
a)
b)
Checkpoint (page 62)
1
a)
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
3
© Jing Kung. All rights reserved.
New 21st Century Chemistry
b)
c)
d)
2
a)
b)
c)
Checkpoint (page 66)
1
a)
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
4
© Jing Kung. All rights reserved.
New 21st Century Chemistry
b)
c)
2
a)
b)
Checkpoint (page 68)
1 a) The boiling point of a compound depends on the strength of its intermolecular attractions.
Molecule of hexane is longer and somewhat spreadout while that of 2,2-dimethylbutane is
more spherical and compact.
The shape of molecule of hexane allows greater surface contact between molecules.
Thus the van der Waals’ forces in hexane are stronger than that in 2,2-dimethylbutane.
Hence the boiling point of hexane is higher than that of 2,2-dimethylbutane.
b) 2-methylpentane has a structure in between the linear structure of hexane and the spherical
structure of 2,2-dimethylbutane.
The van der Waals’ forces in 2-methylpentane are probably in between those in hexane and
2,2-dimethylbutane.
Hence the boiling point of 2-methylpentane is probably in between those of hexane and
2,2-dimethylbutane.
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
5
© Jing Kung. All rights reserved.
New 21st Century Chemistry
2
The density of pentane is higher than that of 2,2-dimethylpropane.
Stronger van der Waals’ forces in pentane pull the molecules closer together.
So, the density of pentane is higher.
Unit-end exercises (pages 71 – 77)
Answers for the HKCEE (Paper 1) and HKALE questions are not provided.
3
Name
Molecular formula
Condensed structural formula
Methane
CH4
CH4
Ethane
C2H6
CH3CH3
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
6
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Propane
C3H8
CH3CH2CH3
Butane
C4H10
CH3(CH2)2CH3
Pentane
C5H12
CH3(CH2)3CH3
Hexane
C6H14
CH3(CH2)4CH3
Heptane
C7H16
CH3(CH2)5CH3
Octane
C8H18
CH3(CH2)6CH3
Name
Molecular formula
Condensed structural formula
Ethene
C2H4
CH2=CH2
Propene
C3H6
CH3CH=CH2
But-1-ene
C4H8
CH3CH2CH=CH2
But-2-ene
C4H8
CH3CH=CHCH3
Pent-1-ene
C5H10
CH3CH2CH2CH=CH2
Pent-2-ene
C5H10
CH3CH2CH=CHCH3
4
5
6
7
8
9
a)
b)
c)
d)
B
B
C
F
A and E
D
C (1) Members of some homologous series do NOT contain carbon and hydrogen only. For
example, alkanols contain the –O–H group.
B
Compound
Name
Homologous
series to which
it belongs
State at room
temperature
and pressure
C2H4
ethene
alkene
gas
C3H8
propane
alkene
gas
C4H10
butane
alkene
gas
(1) C2H4 is an alkene while C3H8 and C4H10 are alkanes. The compounds belong to different
homologous series.
10 A (1) Both compounds are alkanes.
(2) Both compounds have the same molecular formula, C4H10.
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
7
© Jing Kung. All rights reserved.
New 21st Century Chemistry
11 a)
b)
c)
e)
d)
f)
g)
i)
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
h)
j)
8
© Jing Kung. All rights reserved.
New 21st Century Chemistry
12
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
9
© Jing Kung. All rights reserved.
New 21st Century Chemistry
13
14 a) propane
b) pentane
c)
d)
e)
f)
g)
h)
15 a)
b)
c)
d)
2-methylbutane
3-ethyl-4-methylheptane
3,3-dimethylpentane
propene
but-2-ene
3-methylpent-1-ene
methylpropan-1-ol
3-ethylpentan-1-ol
butan-2-ol
propanoic acid
e) 5-methylhexanoic acid
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
10
© Jing Kung. All rights reserved.
New 21st Century Chemistry
16 a) i) & ii)
iii) The boiling point of a hydrocarbon increases with the number of carbon atoms in its
iv)
b) i)
ii)
iii)
molecule.
~126 °C
A hydrocarbon is a compound that contains only atoms of carbon and hydrogen.
Carbon dioxide and water
When incomplete combustion occurs, the carbon in the hydrocarbons forms carbon
monoxide.
Carbon monoxide is a very poisonous gas.
Suggested answers to in-text activities and unit-end exercises
Topic 7 Unit 26
11
© Jing Kung. All rights reserved.