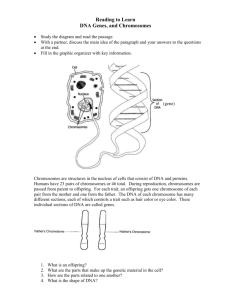
The Process of Science
advertisement

http://highered.mcgraw-hill.com/classware/selfstudy.do?isbn=0072464631 AP biology students this is a collection of notes that I have gathered for you and I have also suggested some videos that you may watch to improve your knowledge of biology. Some of you are having a difficult time taking all the information that is in this course and boiling it down into a simple set of facts, so I have taken the time to produce this resource for you to hopefully make your life easier. :-) When using mastering biology if you want to just read the textbook launch your E text, however if you would like to watch some videos then explore the study area is the link you should click in the opening page of mastering biology. A separate tab should open and from that you should select the chapter guide. The videos in the chapter guide are useful and are commonly of the most important topics within each unit. If you are bored and want to test your knowledge then the self quiz section of each chapter review is useful, I would spend more time gaining knowledge rather than testing it, just a hint. In the notes below I suggest which videos that you should be watching in conjunction with the Reader's Digest version of the text notes. Please note that the text I have highlighted in red is what I consider unimportant for the midyear exam. http://youtu.be/CO-pdIrxZ_w this video explains the mastering biology web site. The Process of Science and how to think like a biologist - this first section is not that important although we need to pay attention to what a controlled experiment is and what a dependent and independent variables are. A good suggestion may be to examine the graph it activity in the themes of study of in the study of life - this is found in the mastering biology. Concept 1.4 GraphIt!: An Introduction to Graphing - since we just finished examining evolution this is a good example of both graphing and how species can introduce themselves into a new habitat. Scientific Method 1. Biology is the scientific study of life, and it consists of many disciplines. 2. The scientific process differs from other ways of learning in that science follows the scientific method, which is characterized by observation, development of a hypothesis, experimentation and data collection, and forming a conclusion. B. Observation 1. Scientists believe nature is orderly and measurable, and that natural laws (e.g., gravity) do not change with time. 2. Natural events, called, phenomena can therefore be understood from observations. 3. Scientists also use the knowledge and experiences of other scientists to expand their understanding of phenomena. 4. Chance alone can sometimes help a scientist get an idea (e.g., Alexander Fleming's discovery of penicillin). C. Hypothesis 1. Inductive reasoning allows a person to combine isolated facts into a cohesive whole. 2. A scientist uses inductive reasoning to develop a possible explanation (a hypothesis) for a natural event; the scientist presents the hypothesis as an actual statement. 3. Scientists only consider hypotheses that can be tested (i.e., moral and religious beliefs may not be testable by the scientific method). D. Experiments/Further Observations 1. Testing a hypothesis involves either conducting an experiment or making further observations. 2. Deductive reasoning involves "if, then" logic to make a prediction that the hypothesis can be supported by experimentation. 3. An experimental design is proposed to test the hypothesis in a meaningful way. 4. An experiment should include a control group which goes through all the steps of an experiment but lacks (or is not exposed to) the factor being tested. 5. Scientists may use a model (a representation of an actual object) in their experiments. 6. Results obtained from use of a model will remain a hypothesis in need of testing if it is impossible to test the actual phenomenon. E. Data 1. Data are the results of an experiment, and are observable and objective rather than subjective. 2. Data are often displayed in a graph or table. 3. Many studies rely on statistical data which, among other things, determines the probability of error in the experiment. F. Conclusion 1. Whether the data support or reject the hypothesis is the basis for the conclusion. 2. The conclusion of one experiment can lead to the hypothesis for another experiment. 3. Scientists report their findings in scientific journals so that their methodology and data are available to other scientists. 4. The experiments and observations must be repeatable or the research is suspect. G. Scientific Theory 1. The ultimate goal is to understand the natural world in scientific theories, which are speculative ideas that join supported, related hypotheses, and are supported by a broad range of observations, experiments, and data. 2. Some basic theories of biology are: a. Cell: all organisms are made of cells. b. Homeostasis: the internal environment of an organism stays relatively constant. c. Gene: organisms contain coded information that dictates their form, function, and behavior. d. Ecosystem: organisms are members of populations which interact with each other and the physical environment. e. Evolution: all living things have a common ancestor. 3. A principle or a law is a theory that is generally accepted by most scientists. H. A Controlled Study 1. A controlled study ensures that the outcome is due to the experimental (independent) variable, the factor being tested. 2. The result is called the responding (dependent) variable because it is due to the dependent variable. 3. The Experiment a. Hypothesis: pigeon pea/winter wheat rotation will increase winter wheat production as well as or better than the use of nitrogen fertilizer. b. Prediction: wheat biomass following the growth of pigeon peas in the soil will surpass wheat biomass following nitrogen fertilizer treatment. c. Control group: winter wheat that receives no fertilizer. d. Test groups: winter wheat treated with different levels of fertilizer; winter wheat grown in soil into which pigeon pea plants had been tilled. e. Environmental conditions and watering were identical in control and test groups. f. Results: all test groups produced more biomass than control group, but high level of nitrogen fertilizer produced more biomass than pigeon pea test group. Thus, hypothesis is not supported. 4. Continuing the Experiment a. To test the hypothesis that pigeon pea residues will build up over time and will increase winter wheat production compared to nitrogen fertilizer, the study is continued for another year. b. The fertilizer-only treatment no longer exceeded biomass production with the use of pigeon peas; biomass in the pigeon pea-treated test group was highest. c. Conclusion: at the end of two years, the yield of winter wheat is better in the pigeon peatreated test group. Hypothesis supported. d. Continuation of the study for another year showed that the soil was continuously improved by the pigeon peas compared to the nitrogen fertilizer test groups. e. Results were reported in a scientific journal. I. A Field Study 1. Hypothesis: aggression of the male mountain bluebird varies during the reproductive cycle. 2. Prediction: aggression will change after the nest is built, after the first egg is laid, and after hatching. 3. To test the hypothesis, a male bluebird model was placed near the nest while the male was gone and observations were made upon his return. 4. Control: a model of a male robin placed near certain nests. 5. Results: resident male bluebirds did not bother the control model but were aggressive toward the male bluebird model depending on the stage in the reproductive cycle. 6. Conclusion: hypothesis is supported. 7. Study was reported in scientific journal with evolutionary interpretation. Chapters 2 and 3 - chemistry of life and properties of water in living things. For these two chapters I would concentrate on the activities related to bonding and the properties of water. 2 - Chemical Elements 1. 2. 3. 4. Matter is defined as anything that takes up space and has mass. Matter exists in three states: solid, liquid, and gas. All matter (both living and non-living) is composed of 92 naturally-occurring elements. Elements, by definition, cannot be broken down to simpler substances with different chemical or physical properties. 5. Six elements (carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur—acronym CHNOPS) make up 98% of the body weight of organisms. B. Atomic Structure 1. Elements consist of tiny particles called atoms. 2. An atom is the smallest unit of an element that displays the properties of the element. 3. One or two letters (e.g., H, Na) create the atomic symbol of the element. 4. The atomic mass of an atom depends on the presence of certain subatomic particles. a. Atoms contain specific numbers of protons, neutrons, and electrons. b. Protons and neutrons are in the nucleus of an atom; electrons move around the nucleus. c. Protons are positively charged particles; neutrons have no charge; both have 1 atomic mass unit (amu) of weight. d. Electrons are negatively charged particles located in orbitals outside the nucleus. 5. All atoms of an element have the same number of protons, called the atomic number of the element. C. The Periodic Table 1. The periodic table shows how various characteristics of atoms of elements recur. 2. Groups are the vertical columns in the table, periods are the horizontal rows; atomic mass increases as you move down a group or across a period. 3. The atomic number is above the atomic symbol and the atomic mass is below the atomic symbol. D. Isotopes 1. Isotopes are atoms of the same element that differ in the number of neutrons (and therefore have different atomic masses). For example, carbon-12 has 6 protons and 6 neutrons, carbon-14 has 6 protons and 8 neutrons. 2. A carbon atom with 8 rather than 6 neutrons is unstable; it releases energy and subatomic particles and is thus a radioactive isotope. 3. Because the chemical behavior of a radioactive isotope is the same as a stable isotope of a particular element, low levels of the radioactive isotope (e.g., radioactive iodine or glucose) allow researchers to trace the location and activity of the element in living tissues; these isotopes are called tracers. 4. High levels of radiation can destroy cells and cause cancer; careful use of radiation can sterilize products and kill cancer cells. E. Electrons and Energy 1. Electrons occupy orbitals within various energy levels (or electron shells) near or distant from the nucleus of the atom. The farther the orbital from the nucleus, the higher the energy level. 2. An orbital is a volume of space where an electron is most likely to be found; an orbital can contain no more than 2 electrons. 3. When atoms absorb energy during photosynthesis, electrons are boosted to higher energy levels. When the electrons return to their original energy level, the released energy is converted into chemical energy. This chemical energy supports all life on Earth. 4. The innermost shell of an atom is complete with 2 electrons; all other shells are complete with 8 electrons. This is called the octet rule. 5. Atoms will give up, accept, or share electrons in order to have 8 electrons in a electron shell. Elements and Compounds 1. When atoms of two or more different elements bond together, they form a compound (e.g., H2O). 2. A molecule is the smallest part of a compound that has the properties of the compound. 3. Electrons possess energy, and bonds that exist between atoms in molecules therefore contain energy. B. Ionic Bonding 1. An ionic bond forms when electrons are transferred from one atom to another atom. 2. By losing or gaining electrons, atoms fill outer shells, and are more stable (the octet rule). 3. Example: sodium loses an electron and therefore has a positive charge; chlorine gains an electron to give it a negative charge. Such charged particles are called ions. 4. Attraction of oppositely charged ions holds the two atoms together in an ionic bond. 5. A salt (e.g., NaCl) is an example of an ionically-bonded compound. C. Covalent Bonding 1. Covalent bonds result when two atoms share electrons so each atom has an octet of electrons in the outer shell (or, in the case of hydrogen, 2 electrons). 2. Hydrogen can give up an electron to become a hydrogen ion (H+) or share an electron with another atom to complete its shell with 2 electrons. 3. The structural formula of a compound indicates a shared pair of electrons by a line between the two atoms; e.g., single covalent bond (H–H), double covalent bond (O=O), and triple covalent bond (N = N). Each line between the atoms represents a pair of electrons. 4. The three-dimensional shapes of molecules are not represented by structural formulas, but shape is critical in understanding the biological action of molecules. Different molecules have different three-dimensional shapes, depending on the number of atoms in the molecule and the types of bonds (single , double, or triple covalent). D. Nonpolar and Polar Covalent Bonds 1. In nonpolar covalent bonds, sharing of electrons is equal, i.e., the electrons are not attracted to either atom to a greater degree. 2. With polar covalent bonds, the sharing of electrons is unequal. a. In a water molecule (H2O), sharing of electrons by oxygen and hydrogen is not equal; the oxygen atom with more protons attracts the electrons closer to it, and thus dominates the H2O association. b. Attraction of an atom for electrons in a covalent bond is called the electronegativity of the atom; an oxygen atom is more electronegative than a hydrogen atom. c. Oxygen in a water molecule, more attracted to the electron pair, assumes a partial negative charge. E. Hydrogen Bonding 1. A hydrogen bond is a weak attractive force between the slightly positive charge of the hydrogen atom of one molecule and slightly negative charge of another atom (e.g., oxygen, nitrogen) in another or the same molecule. 2. Many hydrogen bonds taken together are relatively strong. 3. Hydrogen bonds between and within complex biological molecules (e.g., DNA, proteins) help maintain their proper structure and function. 3 - Chemistry of Water 1. All living things are 70–90% water. 2. Because water is a polar molecule, water molecules are hydrogen bonded to one other. 3. Because of hydrogen bonding, water is liquid between 0° C and 100° C which is essential for the existence of life. B. Properties of Water 1. Water has a high heat capacity a. The temperature of liquid water rises and falls more slowly than that of most other liquids. b. A calorie is the amount of heat energy required to raise the temperature of one gram of water 1° C. c. Because the hydrogen bonds between water molecules hold more heat, water's temperature falls more slowly than other liquids; this protects organisms from rapid temperature changes and helps them maintain homeostatic temperature. 2. Water has a high heat of vaporization. a. Hydrogen bonds between water molecules require a relatively large amount of heat to break. b. This property moderates Earth's surface temperature; permits living systems to exist. c. When animals sweat, evaporation of the sweat removes body heat, thus cooling the animal. 3. Water is a solvent. a. Water dissolves a great number of substances (e.g., salts, large polar molecules). b. Ionized or polar molecules attracted to water are hydrophilic ("water loving"). c. Nonionized and nonpolar molecules that cannot attract water are hydrophobic ("water fearing"). d. A solution contains dissolved substances called solutes. 4. Water molecules are cohesive and adhesive. a. Cohesion allows water to flow freely without molecules separating. b. Adhesion is ability to adhere to polar surfaces; water molecules have positive and negative poles. c. Water rises up a tree from roots to leaves through small tubes. i. Adhesion of water to walls of vessels prevents water column from breaking apart. ii. Cohesion allows evaporation from leaves to pull water column from roots. 5. Water has a high surface tension. a. Water is relatively difficult to break through at its surface. b. This property permits a rock to be skipped across a pond surface, and supports insects walking on surface. 6. Unlike most substances, frozen water is less dense than liquid water. a. Below 4° C, hydrogen bonding becomes more rigid but more open, causing expansion. b. Because ice is less dense, it floats; therefore, bodies of water freeze from the top down. c. If ice was heavier than water, ice would sink and bodies of water would freeze solid. d. This property allows ice to act as an insulator on bodies of water, thereby protecting aquatic organisms during the winter. C. Acids and Bases 1. When water ionizes or dissociates, it releases a small (107 moles/liter) but equal number of hydrogen (H+) ions and hydroxide (OH-) ions; H – O –H → H+ + OH-. 2. Acid molecules dissociate in water, releasing hydrogen (H+) ions: HCl → H+ + Cl-. 3. Bases are molecules that take up hydrogen ions or release hydroxide ions. NaOH → Na+ + OH-. 4. The pH scale indicates acidity and basicity (alkalinity) of a solution. a. pH is the measurement of free hydrogen ions, expressed as a negative logarithm of the H+ concentration (-log [H+]). b. pH values range from 0 (100 moles/liter; most acidic) to 14 (1014 moles/liter; most basic). i. One mole of water has 107 moles/liter of hydrogen ions; therefore, has neutral pH of 7. ii. An acid is a substance with pH less than 7; a base is a substance with pH greater than 7. iii. Because it is a logarithmic scale, each lower unit has 10 times the amount of hydrogen ions as next higher pH unit; as move up pH scale, each unit has 10 times the basicity of previous unit. 5. Buffers keep pH steady and within normal limits in living organisms.. a. Buffers stabilize pH of a solution by taking up excess hydrogen (H+) or hydroxide (OH-) ions. b. Carbonic acid helps keep blood pH within normal limits: H2CO3 → H+ + HCO3-. The chemistry section of this course is scary to a lot of students. You need to focus on knowing your functional groups, how carbon acts as a backbone for organic molecules, and you need to know the main groups of biological macromolecules, carbohydrates, lipids, proteins and nucleic acids. 4 - Organic Molecules and Carbon Chemistry Organic molecules contain carbon and hydrogen atoms bonded to other atoms. 1. Four types of organic molecules (biomolecules) exist in organisms: carbohydrates, lipids, proteins, and nucleic acids. 2. Organic molecules are a diverse group; even a simple bacterial cell contains some 5,000 organic molecules. The Carbon Atom 1. The chemictry of the carbon atom allows it to form covalent bonds with as many as four other elements (generally with the CHNOPS elements). 2. Hydrocarbons are chains of carbon atoms bonded exclusively to hydrogen atoms; hydrocarbons can be branched and they can form ringed (cyclic) compounds. 3. Carbon atoms can form double or triple bonds with certain atoms (carbon, nitrogen). A. The Carbon Skeleton and Functional Groups 1. The carbon chain of an organic molecule is called its skeleton or backbone. 2. Functional groups are clusters of specific atoms bonded to the carbon skeleton with characteristic structure and functions. a. As an example, the addition of an –OH (hydroxyl group) to a carbon skeleton turns the molecule into an alcohol. b. Ethyl alcohol (ethanol) is hydrophilic (dissolves in water) because the hydroxyl group is polar. c. Nonpolar organic molecules are hydrophobic (cannot dissolve in water) unless they contain a polar functional group. An example is ethane. d. Depending on its functional groups, an organic molecule may be both acidic and hydrophilic. An example is a hydrocarbon that contains a carboxyl group; carboxyl groups ionize in solution by releasing hydrogen ions, becoming both polar and acidic. e. Because cells are 70–90% water, the degree to which an organic molecule interacts with water affects its function. 3. Isomers are molecules with identical molecular formulas but different arrangements of their atoms (e.g., glyceraldehyde and dihydroxyacetone). B. The Macromolecules of Cells 1. Carbohydrates, lipids, proteins, and nucleic acids are called macromolecules because of their large size. 2. The largest macromolecules are called polymers, constructed by linking many of the same type of small subunits, called monomers. Examples: amino acids (monomers) are linked to form a protein (polymer); many nucleotides (monomers) are linked to form a nucleic acid (polymer). 3. Cellular enzymes carry out dehydration reactions to synthesize macromolecules. In a dehydration reaction, a water molecule is removed and a covalent bond is made between two atoms of the monomers. a. In a dehydration reaction, a hydroxyl (—OH) group is removed from one monomer and a hydrogen (—H) is removed from the other. b. This produces water, and, because the water is leaving the monomers, it is a dehydration reaction. 4. Hydrolysis ("water breaking") reactions break down polymers in reverse of dehydration; a hydroxyl (—OH) group from water attaches to one monomer and hydrogen (—H) attaches to the other. 5. Enzymes are molecules that speed up chemical reactions by bringing reactants together; an enzyme may even participate in the reaction but is not changed by the reaction. Carbohydrates A. Monosaccharides: Ready Energy 1. Monosaccharides are simple sugars with a backbone of 3 to 7 carbon atoms. a. Most monosaccharides of organisms have 6 carbons (hexose). b. Glucose and fructose are hexoses, but are isomers of one another; each has the formula (C6H12O6) but they differ in arrangement of the atoms. c. Glucoseis found in the blood of animals; it is the source of biochemical energy (ATP) in nearly all organisms. 2. Ribose and deoxyribose are five-carbon sugars (pentoses); they contribute to the backbones of RNA and DNA, respectively. B. Disaccharides: Varied Uses 1. Disaccharides contain two monosaccharides joined by a dehydration reaction. 2. Lactose is composed of galactose and glucose and is found in milk. 3. Maltose is composed of two glucose molecules; it forms in the digestive tract of humans during starch digestion. 4. Sucrose (table sugar) is composed of glucose and fructose; it is used to sweeten food for human consumption. C. Polysaccharides as Energy Storage Molecules 1. Polysaccharides are polymers of monosaccharides. They are not soluble in water and do not pass through the plasma membrane of the cell. 2. Starch, found in many plants, is a straight chain of glucose molecules with relatively few side branches. Amylose and amylopectin are the two forms of starch found in plants. 3. Glycogen is a highly branched polymer of glucose with many side branches. It is the storage form of glucose in animals. D. Polysaccharides as Structural Molecules 1. Cellulose is a polymer of glucose which forms microfibrils, the primary constituent of plant cell walls. a. Cotton is nearly pure cellulose. b. Cellulose is indigestible by humans due to the unique bond between glucose molecules. c. Grazing animals can digest cellulose due to special stomachs and bacteria. d. Cellulose is the most abundant organic molecule on Earth. 2. Chitin is a polymer of glucose with an amino group attached to each glucose. a. Chitin is the primary constituent of the exoskeleton of crabs and related animals (lobsters, insects, etc.). b. Chitin is not digestible by humans. Lipids Lipids are varied in structure. 1. Lipids are hydrocarbons that are insoluble in water because they lack polar groups. 2. Fat provides insulation and energy storage in animals. 3. Phospholipids form plasma membranes and steroids are important cell messengers. 4. Waxes have protective functions in many organisms. A. Triglycerides: Long-Term Energy Storage 1. Fats and oils contain two molecular units: glycerol and fatty acids. 2. Glycerol is a water-soluble compound with three hydroxyl groups. 3. Triglycerides are glycerol joined to three fatty acids by dehydration reactions. 4. A fatty acid is a long hydrocarbon chain with a carboxyl (acid) group at one end. a. Most fatty acids in cells contain 16 to 18 carbon atoms per molecule. b. Saturated fatty acids have no double bonds between their carbon atoms. c. Unsaturated fatty acids have double bonds in the carbon chain where there are less than two hydrogens per carbon atom. 5. Fats contain saturated fatty acids and are solid at room temperature (e.g., butter). 6. Oils contain unsaturated fatty acids and are liquid at room temperature. 7. Animals use fat rather than glycogen for long-term energy storage; fat stores more energy. B. Phospholipids: Membrane Components 1. Phospholipids are constructed like neutral fats except that the third fatty acid is replaced by a polar (hydrophilic) phosphate group; the phosphate group usually bonds to another organic group (designated by R). 2. The hydrocarbon chains of the fatty acids become the nonpolar (hydrophobic) tails. 3. Phospholipids arrange themselves in a double layer in water, so the polar heads face toward water molecules and nonpolar tails face toward one other, away from water molecules. 4. This property enables phospholipids to form an interface or separation between two solutions (e.g., the interior and exterior of a cell); the plasma membrane is a phospholipid bilayer. C. Steroids: Four Fused Rings 1. Steroids have skeletons of four fused carbon rings and vary according to attached functional groups; these functional groups determine the biological functions of the various steroid molecules. 2. Cholesterol is a component of an animal cell's plasma membrane, and is the precursor of the steroid hormone (aldosterone, testosterone, estrogen, calcitriol, etc.). 3. A diet high in saturated fats and cholesterol can lead to circulatory disorders. D. Waxes 1. Waxes are long-chain fatty acids bonded to long-chain alcohols. 2. Waxes have a high melting point, are waterproof, and resist degradation. 3. Waxes form a protective covering in plants that retards water loss in leaves and fruits. 4. In animals, waxes maintain animal skin and fur, trap dust and dirt, and form the honeycomb. Proteins Protein Functions 1. Support proteins include keratin, which makes up hair and nails, and collagen fibers, which support many of the body's structures (e.g., ligaments, tendons, skin). 2. Enzymes are proteins that act as organic catalysts to accelerate chemical reactions within cells. 3. Transport functions include channel and carrier proteins in the plasma membrane, and hemoglobin that transports oxygen in red blood cells. 4. Defense functions include antibodies that prevent infection. 5. Hormones are regulatory proteins that influence the metabolism of cells. For example, insulin regulates glucose content of blood and within cells. 6. Motion within cells and by muscle contraction is provided by the proteins myosin and actin. A. Amino Acids: Building Blocks of Proteins 1. Amino acids contain an acidic group (— COOH) and an amino group (—NH2). 2. Amino acids differ according to their particular R group, ranging from single hydrogen to complicated ring compounds. 3. The R group of amino acid cystine ends with a sulfhydryl (—SH) that serves to connect one chain of amino acids to another by a disulfide bond (— S—S—). 4. There are 20 different amino acids commonly found in cells. B. Peptides 1. A peptide bond is a covalent bond between two amino acids. 2. Atoms of a peptide bond share electrons unevenly (oxygen is more electronegative than nitrogen). 3. The polarity of the peptide bond permits hydrogen bonding between different amino acids in a polypeptide. 4. A peptide is two or more amino acids bonded together. 5. Polypeptides are chains of many amino acids joined by peptide bonds. 6. A protein may contain more than one polypeptide chain; it can thus have a very large number of amino acids. a. The three-dimensional shape of a protein is critical; an abnormal sequence will have the wrong shape and will not function normally. b. Frederick Sanger determined the first protein sequence (of the hormone insulin) in 1953. C. Shape of Proteins 1. Protein shape determines the function of the protein in the organism; proteins can have up to four levels of structure (but not all proteins have four levels). 2. The primary structure is the protein's own particular sequence of amino acids. a. Just as the English alphabet contains 26 letters, 20 amino acids can join to form a huge variety of "words." 3. The secondary structure results when a polypeptide coils or folds in a particular way. a. The a (alpha) helix was the first pattern discovered. i. In a peptide bond, oxygen is partially negative, hydrogen is partially positive. ii. This allows for hydrogen bonding between the C=O of one amino acid and the N—H of another. iii. Hydrogen bonding between every fourth amino acid holds the spiral shape of an a helix. b. The b (beta) sheet was the second pattern discovered. i. Pleated b sheet polypeptides turn back upon themselves. ii. Hydrogen bonding occurs between extended lengths. c. Fibrous proteins (e.g. keratin) are structural proteins with helices and/or pleated sheets that hydrogen bond to one another. 4. Tertiary structure results when proteins are folded, giving rise to the final three-dimensional shape of the protein. This is due to interactions among the R groups of the constituent amino acids. a. Globular proteins tend to ball up into rounded shapes. b. Strong disulfide linkages maintain the tertiary shape; hydrogen, ionic, and covalent bonds also contribute. 5. Quaternary structure results when two or more polypeptides combine. a. Hemoglobin is globular protein with a quaternary structure of four polypeptides; each polypeptide has a primary, secondary, and tertiary structure. D. Protein Folding Diseases 1. As proteins are synthesized, chaperone proteins help them fold into their correct shapes; chaperone proteins may also correct misfolding of a new protein and prevent them from making incorrect shapes. 2. Certain diseases (e.g., the transmissible spongiform encephalopathies, or TSEs) are likely due to misfolded proteins, called prions. Nucleic Acids 1. Nucleic acids are polymers of nucleotides with very specific functions in cells. 2. DNA (deoxyribonucleic acid) stores the genetic code for its own replication and for the amino acid sequences in proteins. 3. RNA (ribonucleic acid) allows for translation of the genetic code of DNA into the amino acid sequence of proteins; other functions for RNA in the cell exist. 4. Some nucleotides have independent metabolic functions in cells. a. Coenzymes are molecules which facilitate enzymatic reactions. b. ATP (adenosine triphosphate) is a nucleotide used to supply energy for synthetic reactions and other energy-requiring metabolic activities in the cell. B. Structure of DNA and RNA 1. Nucleotides are a molecular complex of three types of molecules: a phosphate (phosphoric acid), a pentose sugar, and a nitrogen-containing base. 2. DNA and RNA differ in the following ways: a. Nucleotides of DNA contain deoxyribose sugar; nucleotides of RNA contain ribose. b. In RNA, the base uracil occurs instead of the base thymine. Both RNA and DNA contain adenine, guanine, and cytosine. c. DNA is double-stranded with complementary base pairing; RNA is single-stranded. i. Complementary base pairing occurs where two strands of DNA are held together by hydrogen bonds between purine and pyrimidine bases. ii. The number of purine bases always equals the number of pyrimidine bases. iii. In DNA, thymine is always paired with adenine; cytosine is always paired with guanine. Thus, in DNA: A + G = C + T. d. Two strands of DNA twist to form a double helix; RNA does not form helices. C. ATP (Adenosine Triphosphate) 1. ATP (adenosine triphosphate) is a nucleotide in which adenosine is composed of ribose and adenine. 2. Triphosphate derives its name from three phosphate groups attached together and to the ribose. 3. ATP is a high-energy molecule because the last two phosphate bonds release energy when broken. 4. In cells, the terminal phosphate bond is hydrolyzed, leaving ADP (adenosine diphosphate); energy is released when this occurs. 5. The energy released from ATP breakdown is used in the energy-requiring processes of the cell, such as synthetic reactions, muscle contraction, and the transmission of nerve impulses. The chapter guide of the cell has a lot videos, the most useful videos that I've seen are the tours of the animal and plant cells. Understanding the end of membrane system and how the organelles function together is important understanding how to cell acts as a basic unit of life. The review of animal cell structure and function in the review of plant cell structure and function in concept 6.7 are also very useful. 6 - Cellular Level of Organization 1. Detailed study of the cell began in the 1830s; some of the scientists contributing to the understanding of cell structure and function were Robert Brown, Matthais Schleiden, Theodor Schwann, and Rudolph Virchow. 2. The cell theory states that all organisms are composed of cells, that cells are the structural and functional unit of organisms, and that cells come only from preexisting cells. B. Cell Size 1. Cells range in size from one millimeter down to one micrometer. 2. Cells need a surface area of plasma membrane large enough to adequately exchange materials. 3. The surface-area-to-volume ratio requires that cells be small. a. As cells get larger in volume, surface area relative to volume decreases. b. Size limits how large the actively metabolizing cells can become. c. Cells needing greater surface area utilize membrane modifications such as folding, microvilli, etc. C. Microscopy Today (Science Focus Box) 1. Compound light microscopes use light rays focused by glass lenses. 2. Transmission electron microscopes (TEM) use electrons passing through specimen and focused by magnets. 3. Scanning electron microscopes (SEM) use electrons scanned across metal-coated specimen; secondary electrons given off by metal are collected by a detector. 4. Magnification is a function of wavelength; the shorter wavelengths of electrons allow greater magnification than the longer wavelengths of light rays. 5. Resolution is the minimum distance between two objects at which they can still be seen as separate objects. 6. Immunofluorescence microscopy uses fluorescent antibodies to reveal proteins in cells. 7. Confocal microscopy uses laser beam to focus on a shallow plane within the cell; this forms a series of optical sections from which a computer creates a three dimensional image. 8. Video-enhanced contrast microscopy accentuates the light and dark regions and may use a computer to contrast regions with false colors. 9. Bright-field, phase contrast, differential interference, and darkfield are different types of light microscopes. Prokaryotic Cells 1. Prokaryotic cells lack a nucleus and are smaller and simpler than eukaryotic cells (which have a nucleus). 2. Prokaryotic cells are placed in two taxonomic domains: Bacteria and Archaea. Organisms in these two domains are structurally similar but biochemically different. B. The Structure of Bacteria 1. Bacteria are extremely small; average size is 1–1.5 μm wide and 2–6 μm long . 2. Bacteria occur in three basic shapes: spherical coccus, rod-shaped bacillus, and spiral spirillum (if rigid) or spirochete (if flexible). 3. Cell Envelope a. Includes the plasma membrane, the cell wall, and the glycocalyx. The plasma membrane is a lipid bilayer with imbedded and peripheral proteins; it regulates the movement of substances into and out of the cell. b. The plasma membrane can form internal pouches called mesosomes, which increase the internal surface area of the membrane for enzyme attachment. c. The cell wall maintains the shape of the cell and is strengthened by peptidoglycan. d. The glycocalyx is a layer of polysaccharides on the outside of the cell wall; it is called a capsule if organized and not easily removed, or a slime layer if it is not well-organized and is easily removed. 4. Cytoplasm a. The cytoplasm is a semifluid solution containing water, inorganic and organic molecules, and enzymes. b. The nucleoid is a region that contains the single, circular DNA molecule. c. Plasmids are small accessory (extrachromosomal) rings of DNA; they are not part of the bacterial genetic material. d. Ribosomes are particles with two RNA- and protein-containing subunits that synthesize proteins. e. Inclusion bodies in the cytoplasm are granules of stored substances. f. Cyanobacteria (also called blue-green bacteria) are bacteria that photosynthesize; they lack chloroplasts but have thylakoids containing chlorophyll and other pigments. 5. Appendages a. Motile bacteria usually have flagella; the filament, hook, and basal body work to rotate the flagellum like a propeller to move through fluid medium. b. Fimbriae are small, bristlelike fibers that attach to an appropriate surface. c. Sex pili are tubes used by bacteria to pass DNA from cell to cell. C. The Structure of Archaea 1. In addition to spheres, rods, and spirals, Archaea can be lobed, platelike, or irregular. 2. The cell wall contains various polysaccharides and proteins rather than peptidoglycan. 3. The membrane lipids are composed of glycerol bonded to hydrocarbons, not fatty acids. 4. The DNA and RNA base sequences are closer to eukaryotes than bacteria. 5. Many Archaea are found in extremely salty or hot environments; they may have been the first type of cell to evolve. Eukaryotic Cells 1. Eukaryotic cells are members of the domain Eukarya, which includes the protists, fungi, plants, and animals. 2. A membrane-bounded nucleus houses DNA; the nucleus may have originated as an invagination of the plasma membrane. 3. Eukaryotic cells are much larger than prokaryotic cells, and therefore have less surface area per volume. 4. Eukaryotic cells are compartmentalized; they contain small structures called organelles that perform specific functions. 5. Some eukaryotic cells (e.g., plant cells) have a cell wall containing cellulose; plasmodesmata are channels in a cell wall that allow cytoplasmic strands to extend between adjacent cells. B. The Structure of Eukaryotic Cells 1. The nucleus communicates with ribosomes in the cytoplasm. 2. The organelles of the endomembrane system communicate with one another; each organelle contains its own set of enzymes and produces its own products, which move from one organelle to another by transport vesicles. 3. The energy-related mitochondria (plant and animal cells) and chloroplasts (plant cells) do not communicate with other organelles; they contain their own DNA and are self-sufficient. 4. The cytoskeleton is a lattice of protein fibers that maintains the shape of the cell and assists in movement of the organelles. C. Cell Fractionation and Differential Centrifugation (Science Focus Box) 1. Cell fractionation allows the researcher to isolate and individually study the organelles of a cell. 2. Differential centrifugation separates the cellular components by size and density. 3. Using these two techniques, researchers can obtain pure preparations of any cell component. D. The Nucleus and Ribosomes 1. The nucleus has a diameter of about 5 μm. 2. Chromatin is a threadlike material that coils into chromosomes just before cell division occurs; contains DNA, protein, and some RNA. 3. Nucleoplasm is the semifluid medium of the nucleus. 4. Chromosomes are rodlike structures formed during cell division; composed of coiled or folded chromatin. 5. The nucleolus is a dark region of chromatin inside the nucleus; it is the site where ribosomal RNA (rRNA) joins with proteins to form ribosomes. 6. The nucleus is separated from the cytoplasm by the nuclear envelope, which contains nuclear pores to permit passage of substances (e.g., ribosomal subunits, messenger RNA, proteins, etc.) in and out of the nucleus 7. Ribosomes are the site of protein synthesis in the cell. In eukaryotic cells, ribosomes may occur freely or in groups called polyribosomes. 8. Ribosomes receive messenger RNA (mRNA) from the nucleus, which instructs the ribosomes of the correct sequence of amino acids in a protein to be synthesized. E. The Endomembrane System 1. The endomembrane system is a series of intracellular membranes that compartmentalize the cell. 2. It consists of the nuclear envelope, the membranes of the endoplasmic reticulum, the Golgi apparatus, and several types of vesicles. 3. Endoplasmic Reticulum a. The endoplasmic reticulum (ER) is a system of membrane channels and saccules (flattened vesicles) continuous with the outer membrane of the nuclear envelope. b. Rough ER is studded with ribosomes on the cytoplasm side; it is the site where proteins are synthesized and enter the ER interior for processing and modification. c. Smooth ER is continuous with rough ER but lacks ribosomes; it is a site of various synthetic processes, detoxification, and storage; smooth ER forms transport vesicles. 4. The Golgi Apparatus a. It is named for Camillo Golgi, who discovered it in 1898. b. The Golgi apparatus consists of a stack of slightly curved saccules. c. The Golgi apparatus receives protein-filled vesicles that bud from the rough ER and lipid-filled vesicles from the smooth ER. d. Enzymes within the Golgi apparatus modify the carbohydrates that were placed on proteins in the ER; proteins and lipids are sorted and packaged. e. Vesicles formed from the membrane of the outer face of the Golgi apparatus move to different locations in a cell; at the plasma membrane they discharge their contents as secretions, a process called exocytosis because substances exit the cell. 5. Lysosomes a. Lysosomes are membrane-bounded vesicles produced by the Golgi apparatus. b. Lysosomes contain powerful digestive enzymes and are highly acidic. c. Macromolecules enter a cell by vesicle formation; lysosomes fuse with vesicles and digest the contents of the vesicle. d. White blood cells that engulf bacteria use lysosomes to digest the bacteria. e. Autodigestion occurs when lysosomes digest parts of cells. f. Lysosomes participate in apoptosis, or programmed cell death, a normal part of development. 6. Endomembrane System Summary a. Proteins produced in rough ER and lipids from smooth ER are carried in vesicles to the Golgi apparatus. b. The Golgi apparatus modifies these products and then sorts and packages them into vesicles that go to various cell destinations. c. Secretory vesicles carry products to the membrane where exocytosis produces secretions. d. Lysosomes fuse with incoming vesicles and digest macromolecules. F. Peroxisomes and Vacuoles 1. Peroxisomes are membrane-bounded vesicles that contain specific enzymes. a. Peroxisome action results in production of hydrogen peroxide. b. Hydrogen peroxide (H2O2) is broken down to water and oxygen by catalase. c. Peroxisomes in the liver produce bile salts from cholesterol and also break down fats. d. Peroxisomes also occur in germinating seeds where they convert oils into sugars used as nutrients by the growing plant. 2. Vacuoles a. Vacuoles are mebranous sacs and are larger than vesicles. b. Contractile vacuoles in some protists rid the cell of excess water. c. Digestive vacuoles digest nutrients. d. Vacuoles generally store substances, e.g., plant vacuoles contain water, sugars, salts, pigments, and toxic molecules e. The central vacuole of a plant cell maintins turgor pressure within the cell, stores nutrients and wastes, and degrades organelles as the cell ages. G. Energy-Related Organelles 1. Chloroplasts are membranous organelles (a type of plastid) that serve as the site of photosynthesis. a. Photosynthesis is represented by the equation: b. solar energy + carbon dioxide + water → carbohydrate + oxygen c. Only plants, algae, and certain bacteria are capable of conducting photosynthesis. d. The chloroplast is bound by a double membrane organized into flattened disc-like sacs called thylakoids formed from a third membrane; a stack of thylakoids is a granum. e. Chlorophyll and other pigments capture solar energy, and the enzymes which synthesize carbohydrates are located in the chloroplasts. f. Chloroplasts have both their own DNA and ribosomes, supporting the endosymbiotic hypothesis. g. Other types of plastids, which differ in color, form, and function from chloroplasts, include chromoplasts and leucoplasts. 2. Mitochondria are surrounded by a double membrane: the inner membrane surrounds the matrix and is convoluted to form cristae. a. Mitochondria are smaller than chloroplasts, and often vary their shape. b. Mitochondria also can be fixed in one location or form long, moving chains. c. Mitochondria contain ribosomes and their own DNA. d. The matrix of the mitochondria is concentrated with enzymes that break down carbohydrates. e. ATP production occurs on the cristae. f. More than forty different diseases involving mitochondria have been described. H. The Cytoskeleton 1. The cytoskeleton is a network of connected filaments and tubules; it extends from the nucleus to the plasma membrane in eukaryotes. a. Electron microscopy reveals an organized cytosol. 2. 3. 4. 5. 6. b. Immunofluorescence microscopy identifies protein fibers. c. Elements of the cytoskeleton include: actin filaments, intermediate filaments, and microtubules. Actin Filaments a. Actin filaments are long, thin fibers (about 7 nm in diameter) that occur in bundles or meshlike networks. b. The actin filament consists of two chains of globular actin monomers twisted to form a helix. c. Actin filaments play a structural role, forming a dense complex web just under the plasma membrane; this accounts for the formation of pseudopods in amoeboid movement. d. Actin filaments in microvilli of intestinal cells likely shorten or extend cell into intestine. e. In plant cells, they form tracks along which chloroplasts circulate. f. Actin filaments move by interacting with myosin; myosin combines with and splits ATP, binding to actin and changing configuration to pull actin filament forward. g. Similar action accounts for pinching off cells during cell division. Intermediate Filaments a. Intermediate filaments are 8–11 nm in diameter, between actin filaments and microtubules in size. b. They are rope-like assemblies of fibrous polypeptides. c. Some support the nuclear envelope; others support plasma membrane and form cell-tocell junctions. Microtubules a. Microtubules are small hollow cylinders (25 nm in diameter and from 0.2–25 μm in length). b. Microtubules are composed of a globular protein tubulin that occurs as α tubulin and β tubulin. c. Assembly brings these two together as dimers and the dimers arrange themselves in rows. d. Regulation of microtubule assembly is under control of a microtubule organizing center (MTOC): the main MTOC is called a centrosome. e. Microtubules radiate from the MTOC, helping maintain the shape of cells and acting as tracks along which organelles move. f. Similar to actin-myosin, the motor molecules kinesin and dynein are associated with microtubules. g. Different kinds of kinesin proteins specialize to move one kind of vesicle or cell organelle. h. Cytoplasmic dynein is similar to the molecule dynein found in flagella. Centrioles a. Centrioles are short cylinders with a ring pattern (9 + 0) of microtubule triplets. b. In animal cells and most protists, centrosome contains two centrioles lying at right angles to each other. c. Plant and fungal cells have the equivalent of a centrosome, but they do not contain centrioles. d. Centrioles serve as basal bodies for cilia and flagella. Cilia and Flagella a. Cilia are short, usually numerous hairlike projections that can move in an undulating fashion (e.g., Paramecium; lining of human upper respiratory tract). b. Flagella are longer, usually fewer, projections that move in whip-like fashion (e.g., sperm cells). c. Both have similar construction, but differ from prokaryotic flagella. i. ii. iii. iv. Membrane-bounded cylinders enclose a matrix containing a cylinder of nine pairs of microtubules encircling two single microtubules (9 + 2 pattern of microtubules). Cilia and flagella move when the microtubules slide past one another. Cilia and flagella have a basal body at base with the same arrangement of microtubule triples as centrioles. Cilia and flagella grow by the addition of tubulin dimers to their tips. It is valuable to do the membrane structure activity and watch the bio flicks on membrane transport. Activities on diffusion osmosis and facilitated diffusion are useful reviews for the labs we have conducted on these topics. Be sure to know the difference between active and passive transport and watching or conducting the activities on active transport and exocytosis and endocytosis are useful. 7 - Membrane Models 1. In the early 1900s, researchers noted that lipid-soluble molecules entered cells more rapidly than water-soluble molecules, suggesting lipids are component of plasma membrane. 2. Later, chemical analysis revealed that the membrane contained phospholipids. 3. Gorter and Grendel (1925) found that the amount of phospholipid extracted from a red blood cell was just enough to form one bilayer; they also suggested the nonpolar tails were directed inward and polar heads outward. 4. To account for the permeability of membrane to nonlipid substances, Danielli and Davson (1940s) proposed the "sandwich" model, with a phospholipid bilayer between layers of protein. 5. Robertson (1950s) proposed that proteins were embedded in an outer membrane and that all membranes in cells had similar compositions—the "unit membrane" model. 6. Additional research showed great diversity in membrane structure and function. B. Fluid-Mosaic Model 1. In 1972, Singer and Nicolson introduced the currently accepted fluid-mosaic model. a. The plasma membrane is a phospholipid bilayer, in which protein molecules are embedded. b. Embedded proteins are scattered throughout membrane in an irregular pattern; this varies among membranes. Plasma Membrane Structure and Function 1. The plasma membrane is a phospholipid bilayer with embedded proteins. 2. Phospholipids have both hydrophilic and hydrophobic regions; nonpolar tails (hydrophobic) are directed inward, polar heads (hydrophilic) are directed outward to face both extracellular and intracellular fluid. 3. The proteins form a mosaic pattern on the membrane. 4. Cholesterol is a lipid found in animal plasma membranes; it stiffens and strengthens the membrane. 5. Glycolipids have a structure similar to phospholipids except the hydrophilic head is a variety of sugar; they are protective and assist in various functions. 6. Glycoproteins have an attached carbohydrate chain of sugar that projects externally. 7. The plasma membrane is asymmetrical; glycolipids and proteins occur only on outside and cytoskeletal filaments attach to proteins only on the inside surface. B. Carbohydrate Chains 1. In animal cells, the glycocalyx is a "sugar coat" of carbohydrate chains; it has several functions. 2. Cells are unique in that they have highly varied carbohydrate chains (a "fingerprint"). 3. The immune system recognizes foreign tissues that have inappropriate carbohydrate chains. 4. Carbohydrate chains are the basis for A, B, and O blood groups in humans. C. Fluidity of the Plasma Membrane 1. At body temperature, the phospholipid bilayer has the consistency of olive oil. 2. The greater the concentration of unsaturated fatty acid residues, the more fluid the bilayer. 3. In each monolayer, the hydrocarbon tails wiggle, and entire phospholipid molecules can move sideways. 4. Phospholipid molecules rarely "flip-flop" from one layer to the other. 5. Fluidity of the phospholipid bilayer allows cells to be pliable. 6. Some proteins are held in place by cytoskeletal filaments; most drift in the fluid bilayer. D. The Functions of the Proteins 1. Plasma membrane and organelle membranes have unique proteins; red blood cells (RBC) plasma membrane contains 50+ types of proteins. 2. Membrane proteins determine most of the membrane's functions. 3. Channel proteins allow a particular molecule to cross membrane freely (e.g., Cl-channels). 4. Carrier proteins selectively interact with a specific molecule so it can cross the plasma membrane (e.g., Na+-K+ pump). 5. Cell recognition proteins are glycoproteins that allow the body's immune system to distinguish between foreign invaders and body cells. 6. Receptor proteins are shaped so a specific molecule (e.g., hormone) can bind to it. 7. Enzymatic proteins carry out specific metabolic reactions. Permeability of the Plasma Membrane 1. The plasma membrane is differentially (selectively) permeable; only certain molecules can pass through. a. Small non-charged lipid molecules (alcohol, oxygen) pass through the membrane freely. b. Small polar molecules (carbon dioxide, water) move "down" a concentration gradient, i.e., from high to low concentration. c. Ions and charged molecules cannot readily pass through the hydrophobic component of the bilayer and usually combine with carrier proteins. 2. Both passive and active mechanisms move molecules across membrane. a. Passive transport moves molecules across membrane without expenditure of energy; includes diffusion and facilitated transport. b. Active transport requires a carrier protein and uses energy (ATP) to move molecules across a plasma membrane; includes active transport, exocytosis, endocytosis, and pinocytosis. B. Diffusion and Osmosis 1. Diffusion is the movement of molecules from higher to lower concentration (i.e., "down" the concentration gradient). a. A solution contains a solute, usually a solid, and a solvent, usually a liquid. b. In the case of a dye diffusing in water, the dye is a solute and water is the solvent. c. Once a solute is evenly distributed, random movement continues but with no net change. d. Membrane chemical and physical properties allow only a few types of molecules to cross by diffusion. e. Gases readily diffuse through the lipid bilayer; e.g., the movement of oxygen from air sacs (alveoli) to the blood in lung capillaries depends on the concentration of oxygen in alveoli. f. Temperature, pressure, electrical currents, and molecular size influence the rate of diffusion. 2. Osmosis is the diffusion of water across a differentially (selectively) permeable membrane. a. Osmosis is illustrated by the thistle tube example: i. A differentially permeable membrane separates two solutions. ii. The beaker has more water (lower percentage of solute) and the thistle tube has less water (higher percentage of solute). iii. The membrane does not permit passage of the solute; water enters but the solute does not exit. iv. The membrane permits passage of water with a net movement of water from the beaker to the inside of the thistle tube. b. Osmotic pressure is the pressure that develops in such a system due to osmosis. c. Osmotic pressure results in water being absorbed by the kidneys and water being taken up from tissue fluid. 3. Tonicity is strength of a solution with respect to osmotic pressure. a. Isotonic solutions occur where the relative solute concentrations of two solutions are equal; a 0.9% salt solution is used in injections because it is isotonic to red blood cells (RBCs). b. A hypotonic solution has a solute concentration that is less than another solution; when a cell is placed in a hypotonic solution, water enters the cell and it may undergo cytolysis ("cell bursting"). c. Swelling of a plant cell in a hypotonic solution creates turgor pressure; this is how plants maintain an erect position. d. A hypertonic solution has a solute concentration that is higher than another solution; when a cell is placed in a hypertonic solution, it shrivels (a condition called crenation). e. Plasmolysis is shrinking of the cytoplasm due to osmosis in a hypertonic solution; as the central vacuole loses water, the plasma membrane pulls away from the cell wall. C. Transport by Carrier Proteins 1. The plasma membrane impedes passage of most substances but many molecules enter or leave at rapid rates. 2. Carrier proteins are membrane proteins that combine with and transport only one type of molecule or ion; they are believed to undergo a change in shape to move the molecule across the membrane. 3. Facilitated transport is the transport of a specific solute "down" or "with" its concentration gradient (from high to low), facilitated by a carrier protein; glucose and amino acids move across the membrane in this way. 4. Active transport is transport of a specific solute across plasma membranes "up" or "against" (from low to high) its concentration gradient through use of cellular energy (ATP). a. Iodine is concentrated in cells of thyroid gland, glucose is completely absorbed into lining of digestive tract, and sodium is mostly reabsorbed by kidney tubule lining. b. Active transport requires both carrier proteins and ATP; therefore cells must have high number of mitochondria near membranes where active transport occurs. c. Proteins involved in active transport are often called "pumps"; the sodium-potassium pump is an important carrier system in nerve and muscle cells. d. Salt (NaCl) crosses a plasma membrane because sodium ions are pumped across, and the chloride ion is attracted to the sodium ion and simply diffuses across specific channels in the membrane. 5. Membrane-Assisted Transport a. In exocytosis, a vesicle formed by the Golgi apparatus fuses with the plasma membrane as secretion occurs; insulin leaves insulin-secreting cells by this method. b. During endocytosis, cells take in substances by vesicle formation as plasma membrane pinches off by either phagocytosis, pinocytosis, or receptor-mediated endocytosis. c. In phagocytosis, cells engulf large particles (e.g., bacteria), forming an endocytic vesicle. i. Phagocytosis is commonly performed by ameboid-type cells (e.g., amoebas and macrophages). ii. When the endocytic vesicle fuses with a lysosome, digestion of the internalized substance occurs. d. Pinocytosis occurs when vesicles form around a liquid or very small particles; this is only visible with electron microscopy. e. Receptor-mediated endocytosis, a form of pinocytosis, occurs when specific macromolecules bind to plasma membrane receptors. i. The receptor proteins are shaped to fit with specific substances (vitamin, hormone, lipoprotein molecule, etc.), and are found at one location in the plasma membrane. ii. This location is a coated pit with a layer of fibrous protein on the cytoplasmic side; when the vesicle is uncoated, it may fuse with a lysosome. iii. Pits are associated with exchange of substances between cells (e.g., maternal and fetal blood). iv. This system is selective and more efficient than pinocytosis; it is important in moving substances from maternal to fetal blood. v. Cholesterol (transported in a molecule called a low-density lipoprotein, LDL) enters a cell from the bloodstream via receptors in coated pits; in familial hypocholesterolemia, the LDL receptor cannot bind to the coated pit and the excess cholesterol accumulates in the circulatory system. Modification of Cell Surfaces A. Cell Surfaces in Animals 1. Junctions Between Cells are points of contact between cells that allow them to behave in a coordinated manner. a. Anchoring junctions mechanically attach adjacent cells. b. In adhesion junctions, internal cytoplasmic plaques, firmly attached to cytoskeleton within each cell are joined by intercellular filaments; they hold cells together where tissues stretch (e.g., in heart, stomach, bladder). c. c. In desmosomes, a single point of attachment between adjacent cells connects the cytoskeletons of adjacent cells. d. d. In tight junctions, plasma membrane proteins attach in zipper-like fastenings; they hold cells together so tightly that the tissues are barriers (e.g., epithelial lining of stomach, kidney tubules, blood-brain barrier). e. A gap junction allows cells to communicate; formed when two identical plasma membrane channels join. i. They provide strength to the cells involved and allow the movement of small molecules and ions from the cytoplasm of one cell to the cytoplasm of the other cell. ii. Gap junctions permit flow of ions for heart muscle and smooth muscle cells to contract. 2. The extracellular matrix is a meshwork of polysaccharides and proteins produced by animal cells. a. Collagen gives the matrix strength and elastin gives it resilience. b. Fibronectins and laminins bind to membrane receptors and permit communication between matrix and cytoplasm; these proteins also form "highways" that direct the migration of cells during development. c. Proteoglycans are glycoproteins that provide a packing gel that joins the various proteins in matrix and most likely regulate signaling proteins that bind to receptors in the plasma protein. B. Plant Cell Walls 1. Plant cells are surrounded by a porous cell wall; it varies in thickness, depending on the function of the cell. 2. Plant cells have a primary cell wall composed of cellulose polymers united into threadlike microfibrils that form fibrils. 3. Cellulose fibrils form a framework whose spaces are filled by non-cellulose molecules. 4. Pectins allow the cell wall to stretch and are abundant in the middle lamella that holds cells together. 5. Non-cellulose polysaccharides harden the wall of mature cells. 6. Lignin adds strength and is a common ingredient of secondary cell walls in woody plants. 7. Plasmodesmata are narrow membrane-lined channels that pass through cell walls of neighboring cells and connect their cytoplasms, allowing direct exchange of molecules and ions between neighboring plant cells. The chapter guide for Chapter 8 has a nice MP3 tutor on basic energy concepts. Understanding free energy and how ATP functions in the function of enzymes is also important part of this chapter review of the concepts and the activities in the chapter guide would be useful in helping you understand these concepts. 8 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion; all moving objects have kinetic energy. 3. Potential energy is stored energy. 4. Food is chemical energy; it contains potential energy. 5. Chemical energy can be converted into mechanical energy, e.g., muscle movement. B. Two Laws of Thermodynamics 1. First law of thermodynamics (also called the law of conservation of energy) a. Energy cannot be created or destroyed, but it can be changed from one form to another. b. In an ecosystem, solar energy is converted to chemical energy by the process of photosynthesis; some of the chemical energy in the plant is converted to chemical energy in an animal, which in turn can become mechanical energy or heat loss. c. Neither the plant nor the animal create energy, they convert it from one form to another. d. Likewise, energy is not destroyed; some becomes heat that dissipates into the environment. 2. Second law of thermodynamics a. Energy cannot be changed from one form into another without a loss of usable energy. b. Heat is a form of energy that dissipates into the environment; heat can never be converted back to another form of energy. C. Cells and Entropy 1. Every energy transformation makes the universe less organized and more disordered; entropy is the term used to indicate the relative amount of disorganization. 2. When ions distribute randomly across a membrane, entropy has increased. 3. Organized/usable forms of energy (as in the glucose molecule) have relatively low entropy; unorganized/less stable forms have relatively high entropy. 4. Energy conversions result in heat; therefore, the entropy of the universe is always increasing. 5. Living things depend on a constant supply of energy from the sun, because the ultimate fate of all solar energy in the biosphere is to become randomized in the universe as heat; the living cell is a temporary repository of order purchased at the cost of a constant flow of energy. Metabolic Reactions and Energy Transformations 1. Metabolism is the sum of all the biochemical reactions in a cell. 2. In the reaction A + B = C + D, A and B are reactants and C and D are products. 3. Free energy (DG) is the amount of energy that is free to do work after a chemical reaction. 4. Change in free energy is noted as DG; a negative DG means that products have less free energy than reactants; the reaction occurs spontaneously. 5. Exergonic reactions have a negative DG and energy is released. 6. Endergonic reactions have a positive DG; products have more energy than reactants; such reactions can only occur with an input of energy. Degradative reactions (catabolism) break down molecules; they tend to be exergonic. Synthetic reactions (anabolism) build molecules; they tend to be endergonic. Catabolism - Just as glucose is broken down in cellular respiration, other molecules in the cell undergo catabolism.Fat breaks down into glycerol and three fatty acids. Anabolism - ATP produced during catabolism drives anabolism. Substrates making up pathways can be used as starting materials for synthetic reactions.The molecules used for biosynthesis constitute the cell's metabolic pool. B. ATP: Energy for Cells 1. Adenosine triphosphate (ATP) is the energy currency of cells; when cells need energy, they "spend" ATP. 2. ATP is an energy carrier for many different types of reactions. 3. When ATP is converted into ADP + P, the energy released is sufficient for biological reactions with little wasted. 4. ATP breakdown is coupled to endergonic reactions in a way that minimizes energy loss. 5. ATP is a nucleotide composed of the base adenine and the 5-carbon sugar ribose and three phosphate groups. 6. When one phosphate group is removed, about 7.3 kcal of energy is released per mole. C. Coupled Reactions 1. A coupled reaction occurs when energy released by an exergonic reaction is used to drive an endergonic reaction. 2. ATP breakdown is often coupled to cellular reactions that require energy. 3. ATP supply is maintained by breakdown of glucose during cellular respiration. 4. Only 39% of the chemical energy of glucose is transformed into ATP; 61% is lost as heat. D. ATP can have any of three functions. 1. Chemical Work: ATP supplies energy to synthesize molecules that make up the cell. 2. Transport Work: ATP supplies energy to pump substances across the plasma membrane. 3. Mechanical Work: ATP supplies energy needed to perform mechanical processes (e.g., muscle contraction, propel cilia, etc.). Metabolic Pathways and Enzymes 1. A metabolic pathway is an orderly sequence of linked reactions; each step is catalyzed by a specific enzyme. 2. Metabolic pathways begin with a particular reactant, end with a particular end product(s), and may have many intermediate steps. 3. In many instances, one pathway leads to the next; since pathways often have one or more molecules in common, one pathway can lead to several others. 4. Metabolic energy is captured more easily if it is released in small increments. 5. A reactant is the substance that is converted into a product by the reaction; often many intermediate steps occur. 6. Each step in a series of chemical reactions requires a specific enzyme. 7. Enzymes are catalysts that speed chemical reactions without the enzyme being affected by the reaction. 8. Every enzyme is specific in its action and catalyzes only one reaction or one type of reaction. 9. A substrate is a reactant for an enzymatic reaction. B. Energy of Activation 1. Molecules often do not react with each other unless activated in some way. 2. For metabolic reactions to occur in a cell, an enzyme must usually be present. 3. The energy of activation (Ea) is the energy that must be added to cause molecules to react; without an enzyme (i.e., in a reaction vessel in the laboratory) this energy may be provided by heat, which causes an increase in the number of molecular collisions. C. Enzyme-Substrate Complex 1. Enzymes speed chemical reactions by lowering the energy of activation (Ea) by forming a complex with their substrate(s) at the active site. a. An active site is a small region on the surface of the enzyme where the substrate(s) bind. b. When a substrate binds to an enzyme, the active site undergoes a slight change in shape that facilitates the reaction. This is called the induced fit model of enzyme catalysis. 2. Only a small amount of enzyme is needed in a cell because enzymes are not consumed during catalysis. 3. Some enzymes (e.g., trypsin) actually participate in the reaction. 4. A particular reactant(s) may produce more than one type of product(s). a. Presence or absence of enzyme determines which reaction takes place. b. If reactants can form more than one product, the enzymes present determine which product is formed. 5. Every cell reaction requires its specific enzyme; enzymes are sometimes named for substrates by adding "-ase." D. Factors Affecting Enzymatic Speed 1. Substrate concentration. a. Because molecules must collide to react, enzyme activity increases as substrate concentration increases; as more substrate molecules fill active sites, more product is produced per unit time. 2. Temperature and pH a. As temperature rises, enzyme activity increases because there are more enzyme-substrate collisions. b. Enzyme activity declines rapidly when enzyme is denatured at a certain temperature, due to a change in shape of the enzyme. c. Every enzyme has optimal pH at which its rate of reaction is optimal. d. A change in pH can alter the ionization of the R groups of the amino acids in the enzyme, thereby disrupting the enzyme's activity. 3. Enzyme concentration a. The amount of active enzyme can regulate the rate of an enzymatic reaction. b. Cells can activate specific genes when certain enzymes are needed. c. Enzyme Cofactors i. Many enzymes require an inorganic ion or non-protein cofactor to function. ii. Inorganic cofactors are ions of metals. iii. A coenzyme is an organic cofactor, which assists the enzyme (i.e., it may actually contribute atoms to the reaction). iv. Vitamins are small organic molecules required in trace amounts for synthesis of coenzymes; they become part of a coenzyme's molecular structure; vitamin deficiency causes a lack of a specific coenzyme and therefore a lack of its enzymatic action. v. Phosphorylation of enzymes occurs when signal proteins turn on kinases, which then activate specific enzymes; some hormones use this mechanism. d. Enzyme inhibition occurs when a substance (called an inhibitor) binds to an enzyme and decreases its activity; normally, enzyme inhibition is reversible. i. In competitive inhibition, the substrate and the inhibitor are both able to bind to the enzyme's active site. ii. In noncompetitive inhibition, the inhibitor binds to the enzyme at a location other than the active site (the allosteric site), changing the shape of the enzyme and rendering it unable to bind to its substrate. iii. Competitive and noncompetitive inhibition are both examples of feedback inhibition. iv. In irreversible inhibition, the inhibitor permanently inactivates or destroys the enzyme; cyanide, mercury, and lead are irreversible inhibitors for several specific enzymes. For this chapter I would watch the bio flicks on cellular respiration and do the activity on the overview of cellular respiration. Make sure you understand the concepts of glycolysis, the citric acid cycle and electron transport chain. Make sure you understand that oxygen is the final electron receptor and that there are other metabolic pathways possible when oxygen is not present starting with glycolysis and leading to other metabolic byproducts either alcohol or lactic acid. Please don't be afraid of the detail with these notes, they are excessive and diagrams will replace them and you should use a diagrams of the textbook to understand these processes. Chapter 9 9 - Oxidation-Reduction and the Flow of Energy 1. 2. 3. 4. In oxidation-reduction (redox) reactions, electrons pass from one molecule to another. Oxidation is the loss of electrons. Reduction is the gain of electrons. Both reactions occur at the same time because one molecule accepts electrons given up by another molecule. B. Cellular Respiration 1. The overall equation for cellular respiration is opposite that of photosynthesis: C6H12O6 + 6 O2 = 6 CO2 + 6 H2O + energy 2. When NAD removes hydrogen atoms (H+ + e-) during cellular respiration, the substrate has lost electrons and is therefore oxidized. 3. At the end of cellular respiration, glucose has been oxidized to carbon dioxide and water and ATP molecules have been produced. 4. In metabolic pathways, most oxidations involve the coenzyme NAD+ (nicotinamide adenine dinucleotide); the molecule accepts two electrons but only one hydrogen ion: NAD+ + 2e- + H+ = NADH C. Electron Transport Chain 1. Both photosynthesis and respiration use an electron transport chain consisting of membranebound carriers that pass electrons from one carrier to another. 2. High-energy electrons are delivered to the system and low-energy electrons leave it. 3. The overall effect is a series of redox reactions; every time electrons transfer to a new carrier, energy is released for the production of ATP. D. ATP Production 1. ATP synthesis is coupled to the electron transport system. 2. Peter Mitchell received the 1978 Nobel Prize for his chemiosmotic theory of ATP production. 3. In both mitochondria and chloroplasts, carriers of electron transport systems are located within a membrane. 4. H+ ions (protons) collect on one side of the membrane because they are pumped there by specific proteins. 5. The electrochemical gradient thus established across the membrane is used to provide energy for ATP production. 6. Enzymes and their carrier proteins, called ATP synthase complexes, span the membrane; each complex contains a channel that allows H+ ions to flow down their electrochemical gradient. 7. In photosynthesis, energized electrons lead to the pumping of hydrogen ions across the thylakoid membrane; as hydrogen ions flow through the ATP synthase complex, ATP is formed. 8. During cellular respiration, glucose breakdown provides energy for a hydrogen ion gradient on the inner membrane of the mitochondria that also couples hydrogen ion flow with ATP formation. 9 Cellular Respiration 1. Cellular respiration involves various metabolic pathways that break down carbohydrates and other metabolites with the concomitant buildup of ATP. 2. Cellular respiration consumes oxygen and produces CO2; because oxygen is required, cellular respiration is aerobic. 3. Cellular respiration usually involves the complete breakdown of glucose into CO2 and H2O. 4. The net equation for glucose breakdown is: C6H12O6 + 6 O2 = 6 CO2 + 6 H2O + energy 5. Glucose is a high-energy molecule; CO2 and H2O are low-energy molecules; cellular respiration is thus exergonic because it releases energy. 6. Electrons are removed from substrates and received by oxygen, which combines with H+ to become water. 7. Glucose is oxidized and O2 is reduced. 8. The buildup of ATP is an endergonic reaction (i.e., requires energy). 9. The reactions of cellular respiration allow energy in glucose to be released slowly; therefore ATP is produced gradually. 10. In contrast, if glucose were broken down rapidly, most of its energy would be lost as non-usable heat. 11. The breakdown of glucose yields synthesis of 36 or 38 ATP (depending on certain conditions); this preserves about 39% of the energy available in glucose. 12. This is relatively efficient compared to, for example, the 25% efficiency of a car burning gasoline. B. NAD+ and FAD 1. Each metabolic reaction in cellular respiration is catalyzed by a specific enzyme. 2. As a metabolite is oxidized, NAD+ (nicotinamide adenine dinucleotide) accepts two electrons and a hydrogen ion (H+); this results in NADH + H+. 3. Electrons received by NAD+ and FAD are high-energy electrons and are usually carried to the electron transport chain. 4. NAD+ is a coenzyme of oxidation-reduction since it both accepts and gives up electrons; thus, NAD+ is sometimes called a redox coenzyme 5. Only a small amount of NAD+ is needed in cells because each NAD+ molecule is used repeatedly. 6. FAD coenzyme of oxidation-reduction can replace NAD+; FAD accepts two electrons and two hydrogen ions to become FADH2. C. Phases of Cellular Respiration 1. Cellular respiration includes four phases: a. Glycolysis is the breakdown of glucose in the cytoplasm into two molecules of pyruvate. i. Enough energy is released for an immediate yield of two ATP. ii. Glycolysis takes place outside the mitochondria and does not utilize oxygen; it is therefore an anaerobic process. b. In the preparatory (prep) reaction, pyruvate enters a mitochondrion and is oxidized to a two-carbon acetyl group and CO2 is removed; this reaction occurs twice per glucose molecule. c. The citric acid cycle: i. occurs in the matrix of the mitochondrion and produces NADH and FADH2; ii. is a series of reactions that gives off CO2 and produces one ATP; iii. turns twice because two acetyl-CoA molecules enter the cycle per glucose molecule; iv. produces two immediate ATP molecules per glucose molecule. d. The electron transport chain: i. is a series of carriers in the inner mitochondrial membrane that accept electrons from glucose--electrons are passed from carrier to carrier until received by oxygen; ii. passes electrons from higher to lower energy states, allowing energy to be released and stored for ATP production; iii. accounts for 32 or 34 ATP, depending on certain cell conditions. 2. Pyruvate is a pivotal metabolite in cellular respiration. a. If O2 is not available to the cell, fermentation, an anaerobic process, occurs in the cytoplasm. b. During fermentation, glucose is incompletely metabolized to lactate, or to CO2 and alcohol (depending on the organism). c. Fermentation results in a net gain of only two ATP per glucose molecule. Glycolysis - Outside the Mitochondria: Glycolysis 1. Glycolysis occurs in the cytoplasm outside the mitochondria. 2. Glycolysis is the breakdown of glucose into two pyruvate molecules. 3. Glycolysis is universally found in organisms; therefore, it likely evolved before the citric acid cycle and electron transport chain. B. Energy-Investment Steps 1. Glycolysis begins with the activation of glucose with two ATP; the glucose splits into two C3 molecules known as G3P, each of which carries a phosphate group. C. Energy-Harvesting Steps 1. Oxidation of G3P occurs by removal of electrons and hydrogen ions. 2. Two electrons and one hydrogen ion are accepted by NAD+, resulting in two NADH; later, when the NADH molecules pass two electrons to another electron carrier, they become NAD+ again. 3. The oxidation of G3P and subsequent substrates results in four high-energy phosphate groups, which are used to synthesize four ATP molecules; this process is called substrate-level phosphorylation. 4. Two of four ATP molecules produced are required to replace two ATP molecules used in the initial phosphorylation of glucose; therefore there is a net gain of two ATP from glycolysis. 5. Pyruvate enters a mitochondrion (if oxygen is available) and cellular respiration ensues. 6. If oxygen is not available, fermentation occurs and pyruvate undergoes reduction. The citric acid cycle and the electron transport chain - Inside the Mitochondria 1. The next reactions of cellular respiration involve the preparatoryreaction, the citric acid cycle, and the electron transport chain. 2. In these reactions, the pyruvate from glycolysis is broken down completely to CO2 and H2O. 3. CO2 and ATP are transported out of the mitochondria into the cytoplasm. 4. The H2O can remain in the mitochondria or within the cell, or it can enter the blood and be excreted by the kidneys. 5. A mitochondrion has a double membrane with an intermembrane space (between the outer and inner membrane). 6. Cristae are the inner folds of membrane that jut into the matrix, the innermost compartment of a mitochondrion that is filled with a gel-like fluid. 7. The prep reaction and citric acid cycle enzymes are in the matrix; the electron transport chain is in the cristae. 8. Most of the ATP produced in cellular respiration is produced in the mitochondria; therefore, mitochondria are often called the "powerhouses" of the cell. B. Preparatory Reaction 1. The preparatory reaction connects glycolysis to the citric acid cycle. 2. In this reaction, pyruvate is converted to a two-carbon acetyl group, and is attached to coenzyme A, resulting in the compound acetyl-CoA. 3. This redox reaction removes electrons from pyruvate by a dehydrogenase enzyme, using NAD+ as a coenzyme. 4. This reaction occurs twice for each glucose molecule. C. Citric Acid Cycle 1. The citric acid cycle occurs in the matrix of mitochondria. 2. The cycle is sometimes called the Krebs cycle, named for Sir Hans Krebs, who described the fundamentals of the reactions in the 1930s. 3. The cycle begins by the addition of a two-carbon acetyl group to a four-carbon molecule, forming a six-carbon citrate (citric acid) molecule. 4. In the subsequent reactions, at three different times two electrons and one hydrogen ion are accepted by NAD+, forming NADH. 5. At one time, two electrons and one hydrogen ion are accepted by FAD, forming FADH2. 6. NADH and FADH2 carry these electrons to the electron transport chain. 7. Some energy is released and is used to synthesize ATP by substrate-level phosphorylation. 8. One high-energy metabolite accepts a phosphate group and transfers it to convert ADP to ATP. 9. The citric acid cycle turns twice for each original glucose molecule. 10. The products of the citric acid cycle (per glucose molecule) are 4 CO2, 2 ATP, 6 NADH and 2 FADH2. 11. The six carbon atoms in the glucose molecule have now become the carbon atoms of six CO2 molecules, two from the prep reaction and four from the citric acid cycle. D. The Electron Transport Chain 1. The electron transport chain is located in the cristae of mitochondria and consists of carriers that pass electrons successively from one to another. 2. Some of the protein carriers are cytochrome molecules, complex carbon rings with a heme (iron) group in the center. 3. NADH and FADH2 carry the electrons to the electron transport system.. 4. NADH gives up its electrons and becomes NAD+; the next carrier then gains electrons and is thereby reduced. 5. At each sequential redox reaction, energy is released to form ATP molecules. 6. Because O2 must be present for the proteins to work, this process is also called oxidative phosphorylation. 7. Oxygen serves as the terminal electron acceptor and combines with hydrogen ions to form water. 8. By the time electrons are received by O2, three ATP have been made. 9. When FADH2 delivers electrons to the electron transport system, two ATP are formed by the time the electrons are received by O2. 10. Coenzymes and ATP undergo recycling. a. Cell needs a limited supply of coenzymes NAD+ and FAD because they constantly recycle. b. Once NADH delivers electrons to the electron transport chain, it can accept more hydrogen atoms. c. ADP and phosphate also recycle. d. Efficiency of recycling NAD+, FAD, and ADP eliminates the need to continuously synthesize them anew. E. The Cristae of a Mitochondrion 1. The electron transport chain consists of three protein complexes and two protein mobile carriers that transport electrons. 2. The three protein complexes include NADH-Q reductase complex, the cytochrome reductasecomplex, and the cytochrome oxidase complex; the two protein mobile carriers are coenzyme Q and cytochrome c. 3. Energy released from the flow of electrons down the electron transport chain is used to pump H+ ions, which are carried by NADH and FADH2, into intermembrane space. 4. Accumulation of H+ ions in this intermembrane space creates a strong electrochemical gradient. 5. ATP synthase complexes are channel proteins that serve as enzymes for ATP synthesis. 6. As H+ ions flow from high to low concentration, ATP synthase synthesizes ATP by the reaction: ADP + P = ATP. 7. Chemiosmosis is the term used for ATP production tied to an electrochemical (H+) gradient across a membrane. 8. Respiratory poisons confirm the chemiosmotic nature of ATP synthesis (i.e., a poison that inhibits ATP synthesis increases the H+ gradient). 9. Once formed, ATP molecules diffuse out of the mitochondrial matrix through channel proteins. 10. ATP is the energy currency for all living things; all organisms must continuously produce high levels of ATP to survive. F. Energy Yield From Glucose Metabolism 1. Substrate-Level Phosphorylation a. Per glucose molecule, there is a net gain of two ATP from glycolysis in cytoplasm. b. The citric acid cycle in the matrix of the mitochondria produces two ATP per glucose. c. Thus, a total of four ATP are formed by substrate-level phosphorylation outside of the electron transport chain. 2. Electron Transport Chain and Chemiosmosis a. Most of the ATP is produced by the electron transport chain and chemiosmosis. b. Per glucose, ten NADH and two FADH2 molecules provide electrons and H+ ions to the electron transport chain. c. For each NADH formed within the mitochondrion, three ATP are produced. d. For each FADH2 formed by the citric acid cycle, two ATP are produced. e. For each NADH formed outside mitochondria by glycolysis, two ATP are produced as electrons are shuttled across the mitochondrial membrane by an organic molecule and delivered to FAD. 3. Efficiency of Cellular Respiration a. The energy difference between total reactants (glucose and O2) and products (CO2 and H2O) is 686 kcal. b. An ATP phosphate bond has an energy of 7.3 kcal; 36 to 38 ATP are produced during glucose breakdown for a total of at least 263 kcal. c. This efficiency is 263/686, or 39% of the available energy in glucose is transferred to ATP; the rest of the energy is lost as heat. Fermentation 1. Fermentation is an anaerobic (i.e., occurs in the absence of oxygen) process which consists of glycolysis plus reduction of pyruvate to either lactate or to alcohol and CO2 (depending on the organism). 2. NADH passes its electrons to pyruvate instead of to an electron transport chain; NAD+ is then free to return and pick up more electrons during earlier reactions of glycolysis. 3. Alcoholic fermentation, carried out by yeasts, produces carbon dioxide and ethyl alcohol; this process is used in the production of alcoholic spirits and breads. 4. Lactic acid fermentation, carried out by certain bacteria and fungi, produces lactic acid (lactate); this process is used commercially in the production of cheese, yogurt, and sauerkraut. 5. Other bacteria produce chemicals anaerobically, including isopropanol, butyric acid, proprionic acid, and acetic acid. B. Advantages and Disadvantages of Fermentation 1. Despite a low yield of two ATP molecules, fermentation provides a quick burst of ATP energy for muscular activity. 2. Lactate is toxic to cells. a. When blood cannot remove all lactate from muscles, lactate changes pH and causes muscles to fatigue. b. The individual is in oxygen debt because oxygen is needed to restore ATP levels and rid the body of lactate. c. Recovery occurs after lactate is sent to the liver where it is converted into pyruvate; some pyruvate is then respired or converted back into glucose. C. Efficiency of Fermentation 1. Two ATP produced per glucose molecule during fermentation is equivalent to 14.6 kcal. 2. Complete glucose breakdown to CO2 and H2O during cellular respiration represents a potential yield of 686 kcal of energy. 3. Efficiency of fermentation is 14.6/686 or about 2.1%, far less efficient than complete breakdown of glucose. In studying photosynthesis I would watch the bio flicks on photosynthesis and use the MP3 tutor on photosynthesis. Make sure you know your light reactions and dark reactions that you understand that there are problems when plants make too much oxygen and there are several other pathways the plants use for carbon fixation in hot arid environments. As with the chapter on cellular respiration some of the details within these notes are excessive. The diagrams within the textbook are useful summaries of what is happening during these processes and can replace the detailed notes that are below. 10 Photosynthesis A. Photosynthesis 1. Photosynthesis uses energy to combine carbon dioxide and water to produce glucose in the formula: 6 CO2 + 6 H2O + energy = C6H12O6 + 6 O2 2. When hydrogen atoms are transferred to carbon dioxide from water, water has been oxidized and carbon dioxide has been reduced. 3. Input of energy is needed to produce the high-energy glucose molecule. 4. Chloroplasts capture solar energy and convert it by way of an electron transport system into the chemical energy of ATP. 5. ATP is used along with hydrogen atoms to reduce glucose; when NADP+(nicotinamide adenine dinucleotide phosphate) donates hydrogen atoms (H+ + e-) to a substrate during photosynthesis, the substrate has accepted electrons and is therefore reduced. 6. The reaction that reduces NADP+ is: NADP+ + 2e- + H+ = NADPH 1. Photosynthetic organisms (algae, plants, and cyanobacteria) transform solar energy into carbohydrates. 2. Photosynthetic organisms (plants, algae, cyanobacteria) are called autotrophs because they produce their own food. 3. Organisms that must take in preformed organic molecules are called heterotrophs. 4. Both autotrophs and heterotrophs use organic molecules produced by photosynthesis as chemical building blocks and as a source of energy. B. Flowering Plants as Photosynthesizers 1. Raw materials for photosynthesis are carbon dioxide and water. 2. Roots absorb water that moves up vascular tissue in the stem until it reaches the leaf veins. 3. Carbon dioxide enters a leaf through small openings called stomata. 4. Carbon dioxide and water diffuse into the chloroplasts, the organelles that carry on photosynthesis. 5. In chloroplasts, a double membrane encloses a fluid-filled space called the stroma. 6. An internal membrane system within the stroma forms flattened sacs called thylakoids, which in some cases are organized into stacks to form grana. 7. Spaces within all thylakoids are connected to form an inner compartment, the thylakoid space. 8. Chlorophyll and other pigments involved in absorption of solar energy reside within thylakoid membranes; these pigments absorb solar energy, and energize electrons prior to reduction of CO2 to a carbohydrate. Plants as Solar Energy Converters 1. Only 42% of the solar radiation that hits the Earth's atmosphere reaches surface; most is visible light. 2. Higher energy wavelengths are screened out by the ozone layer in the upper atmosphere. 3. Lower energy wavelengths are screened out by water vapor and CO2. 4. Both the organic molecules within organisms and certain processes (e.g., vision, photosynthesis) are adapted to visible light, the radiation that is most prevalent in the environment. B. Photosynthetic Pigments 1. Photosynthetic pigments use primarily the visible light portion of the electromagnetic spectrum. 2. Pigments found in chlorophyll absorb various portions of visible light; this is called their absorption spectrum. 3. Two major photosynthetic pigments are chlorophyll a and chlorophyll b. 4. Both chlorophylls absorb violet, blue, and red wavelengths best. 5. Very little green light is absorbed; most is reflected (this is why leaves appear green). 6. Carotenoids are yellow-orange pigments that absorb light in violet, blue, and green regions. 7. When chlorophyll breaks down in the fall, the yellow-orange pigments in leaves show through. 8. Absorption and action spectrum a. A spectrophotometer measures the amount of light that passes through a sample. i. As light is shone on a sample, some wavelengths are absorbed and others pass through the sample. ii. A graph of percent of light absorbed at each wavelength is a compound's absorption spectrum. b. Action spectrum i. Photosynthesis produces oxygen; the production rate of oxygen is used to measure the rate of photosynthesis. ii. Oxygen production and therefore photosynthetic activity is measured for plants under each specific wavelength; when plotted on a graph, this gives an action spectrum for a compound. iii. The action spectrum for chlorophyll resembles its absorption spectrum, thus indicating that chlorophyll contributes to photosynthesis. C. Photosynthetic Reaction 1. In 1930, van Niel showed that O2 given off by photosynthesis comes from water and not from CO2. 2. The net equation of photosynthesis reads: 6CO2 + 6H2O = C6 H12O6 + 6O2. D. Two Sets of Reactions 1. In 1905, Blackman proposed two sets of reactions for photosynthesis. 2. Light reactions take place only in the presence of light. a. Light reactions are the energy-capturing reactions. b. Chlorophyl within thylakoid membranes absorbs solar energy and energizes electrons. c. When energized electrons move down an electron transport chain, energy is captured and used for ATP production. d. Energized electrons are also taken up by NADP+, converting it to NADPH. 3. Calvin cycle reactions a. These reactions take place in the stroma; the reactions can occur in either the presence or the absence of light. b. These are synthetic reactions that use NADPH and ATP to reduce CO2. Light Reactions 1. Two electron pathways operate in the thylakoid membrane: the noncyclic pathway and the cyclic pathway. 2. Both pathways produce ATP; only the noncyclic pathway also produces NADPH. 3. ATP production during photosynthesis is called photophosphorylation; therefore these pathways are also known as cyclic and noncyclic photophosphorylation. B. Noncyclic Electron Pathway 1. This pathway occurs in the thylakoid membranes and requires participation of two lightgathering units: photosystem I (PS I) and photosystem II (PS II). 2. A photosystem is a photosynthetic unit comprised of a pigment complex and an electron acceptor; solar energy is absorbed and high-energy electrons are generated. 3. Each photosystem has a pigment complex of chlorophyll a, chlorophyll b, carotenoid, and electron acceptor molecules. 4. Absorbed energy is passed from one pigment molecule to another until concentrated in reactioncenterchlorophyll a molecules. 5. Electrons in reaction-center chlorophyll a become excited, and escape to the electron-acceptor molecule. 6. The noncyclic pathway begins with PSII; electrons move from H2O through PS II to PS I and then on to NADP+. 7. The PS II pigment complex absorbs solar energy; high-energy electrons (e-) leave the reactioncenter chlorophyll a molecule. 8. PS II takes replacement electrons from H2O, which splits, releasing O2 and H+ ions: H2O = 2 H+ + 2 e- + ½ O2. 9. Oxygen is released as oxygen gas (O2). 10. The H+ ions temporarily stay within the thylakoid space and contribute to a H+ ion gradient. 11. As H+ flow down electrochemical gradient through ATP synthase complexes, chemiosmosis occurs. 12. Low-energy electrons leaving the electron transport system enter PS I. 13. When the PS I pigment complex absorbs solar energy, high-energy electrons leave reactioncenter chlorophyll a and are captured by an electron acceptor. 14. The electron acceptor passes them on to NADP+. 15. NADP+ takes on an H+ to become NADPH: NADP+ + 2 e- + H+ = NADPH. 16. NADPH and ATP (produced by noncyclic-flow electrons in the thylakoid membrane) are used by enzymes in the stroma during the light-independent (dark) reactions. C. Cyclic Electron Pathway 1. The cyclic electron pathway begins when the PS I antenna complex absorbs solar energy. 2. High-energy electrons leave PS I reaction-center chlorophyll a molecule. 3. Before they return, the electrons enter and travel down an electron transport chain. a. Electrons pass from a higher to a lower energy level. b. Energy released is stored in the form of a hydrogen (H+) gradient. c. When hydrogen ions flow down their electrochemical gradient through ATP synthase complexes, ATP production occurs. d. The electrons return to PSI rather than move on to NADP+--this is why it is called cyclic and also why no NADPH is produced. 4. It is possible that in plants, the cyclic flow of electrons is utilized only when CO2 is in such limited supply that carbohydrate is not being produced. D. The Organization of the Thylakoid Membrane 1. PS II consists of a pigment complex and electron-acceptor molecules; it oxidizes H2O and produces O2. 2. The electron transport system consists of cytochrome complexes and transports electrons and pumps H+ ions into the thylakoid space. 3. PS I has a pigment complex and electron-acceptor molecules; it is associated with an enzyme that reduces NADP+ to NADPH. 4. ATP synthase complex has an H+ channel and ATP synthase; it produces ATP. E. ATP Production 1. The thylakoid space acts as a reservoir for H+ ions; each time H2O is split, two H+ remain. 2. Electrons move carrier-to-carrier, giving up energy used to pump H+ from the stroma into the thylakoid space. 3. Flow of H+ from high to low concentration across thylakoid membrane provides energy to produce ATP from ADP + P by using an ATP synthase enzyme. 4. This is called chemiosmosis because ATP production is tied to an electrochemical (H+) gradient. Calvin Cycle Reactions 1. The Calvin cycle is a series of reactions producing carbohydrates; these reactions follow the light reactions. 2. The cycle is named for Melvin Calvin who used a radioactive isotope of carbon to trace the reactions. 3. The Calvin cycle includes carbon dioxide fixation, carbon dioxide reduction, and regeneration of ribulose 1,5-bisphosphate (RuBP). B. Fixation of Carbon Dioxide 1. CO2 fixation is the attachment of CO2 to an organic compound called RuBP. 2. RuBP (ribulose bisphosphate) is a five-carbon molecule that combines with carbon dioxide; the resulting 6-carbon molecule then splits into two 3-carbon molecules. 3. The enzyme RuBP carboxylase (rubisco) speeds this reaction; this enzyme comprises 20–50% of the protein content of chloroplasts--it is an unusually slow enzyme. C. Reduction of Carbon Dioxide 1. With the reduction of carbon dioxide, a 3PG (3-phosphoglycerate) molecule forms. 2. Each of two 3PG molecules undergoes reduction to G3P (glyceraldehyde-3-phosphate) in two steps. 3. Light-dependent reactions provide NADPH (electrons) and ATP (energy) to reduce 3PG to G3P. D. Regeneration of RuBP 1. For every three turns of the Calvin cycle, five molecules of G3P are used to re-form three molecules of RuBP. 2. This reaction also uses ATP produced by the light reactions. E. The Importance of the Calvin Cycle 1. G3P, the product of the Calvin Cycle can be converted into many other molecules. 2. Glucose phosphate is one result of G3P metabolism; it is a common energy molecule. 3. Glucose phosphate can bond with fructose to form sucrose. 4. Glucose phosphate is the starting point for synthesis of starch and cellulose. 5. The hydrocarbon skeleton of G3P is used to form fatty acids and glycerol; the addition of nitrogen forms various amino acids. Other Types of Photosynthesis 1. In C3 plants, the Calvin cycle fixes CO2 directly; the first molecule following CO2 fixation is 3PG. 2. In hot weather, stomata close to save water; CO2 concentration decreases in leaves; O2 increases. 3. This is called photorespiration since oxygen is taken up and CO2 is produced; this produces only one 3PG. B. C4 Photosynthesis 1. In a C3 plant, mesophyll cells contain well-formed chloroplasts, arranged in parallel layers. 2. In C4 plants, bundle sheath cells as well as the mesophyll cells contain chloroplasts. 3. In C4 leaf, mesophyll cells are arranged concentrically around the bundle sheath cells. 4. C3 plants use RuBP carboxylase to fix CO2 to RuBP in mesophyll; the first detected molecule is G3P. 5. C4 plants use the enzyme PEP carboxylase (PEPCase) to fix CO2 to PEP (phosphoenolpyruvate, a C3 molecule); the end product is oxaloacetate (a C4 molecule). 6. In C4 plants, CO2 is taken up in mesophyll cells and malate, a reduced form of oxaloacetate, is pumped into the bundle-sheath cells; here CO2 enters Calvin cycle. 7. In hot, dry climates, net photosynthetic rate of C4 plants (e.g., corn) is 2–3 times that of C4 plants. 8. Photorespiration does not occur in C4 leaves because PEPCase does not combine with O2; even when stomates are closed, CO2 is delivered to the Calvin cycle in bundle sheath cells. 9. C4 plants have advantage over C3 plants in hot and dry weather because photorespiration does not occur; e.g., bluegrass (C3) dominates lawns in early summer, whereas crabgrass (C4) takes over in the hot midsummer. C. CAM Photosynthesis 1. CAM (crassulacean-acid metabolism) plants form a C4 molecule at night when stomates can open without loss of water; found in many succulent desert plants including the family Crassulaceae. 2. At night, CAM plants use PEPCase to fix CO2 by forming C4 molecule stored in large vacuoles in mesophyll. 3. C4 formed at night is broken down to CO2 during the day and enters the Calvin cycle within the same cell, which now has NADPH and ATP available to it from the light-dependent reactions. 4. CAM plants open stomates only at night, allowing CO2 to enter photosynthesizing tissues; during the day, stomates are closed to conserve water but CO2 cannot enter photosynthesizing tissues. 5. Photosynthesis in a CAM plant is minimal, due to limited amount of CO2 fixed at night; but this does allow CAM plants to live under stressful conditions. D. Photosynthesis and Adaptation to the Environment 1. Each method of photosynthesis has its advantages, depending on the environment. 2. C4 plants are adapted to areas of high light intensities, high temperatures, and limited rainfall. 3. C3 plants do better in cooler climates. 4. CAM plants do well in an arid environment. For Chapter 12 be sure to watch the bio flicks a mitosis and do the MP3 tutor a mitosis. You may want to conduct the investigation on how much time to sell spending each phase mitosis, make sure you understand that the most time is spent in Interphase of the cell cycle. There are excellent diagrams in the textbook that will replace much of the text within these notes. 12 The Cell Cycle 1. The cell cycle is an orderly set of stages from the first division to the time the daughter cells divide. 2. When a cell is preparing for division, it grows larger, the number of organelles doubles, and the DNA replicates. B. Interphase 1. Most of a cell's life is spent in interphase, in which the cell performs its usual functions. 2. Time spent in interphase varies by cell type: nerve and muscle cells do not complete the cell cycle and remain in the G0 stage while embryonic cells complete the cycle every few hours. 3. The G1 stage is just prior to DNA replication; a cell grows in size, organelles increase in number, and material accumulates for DNA synthesis. 4. The S stage is the DNA synthesis (replication) period; proteins associated with DNA are also synthesized; at the end of the S stage, each chromosome has two identical DNA double helix molecules, called sister chromatids. 5. The G2 stage occurs just prior to cell division; the cell synthesizes proteins needed for cell division, such as proteins in microtubules. 6. Interphase therefore consists of G1, S, and G2. C. M (Mitotic) Stage 1. M stage (M = mitosis) is the entire cell division stage, including both mitosis and cytokinesis. 2. Mitosis is nuclear division, cytokinesis is division of the cytoplasm. 3. When division of the cytoplasm is complete, two daughter cells are produced. D. Control of the Cell Cycle 1. The cell cycle is controlled by both internal and external signals. 2. A signal is a molecule that either stimulates or inhibits a metabolic event. 3. Growth factors are external signals received at the plasma membrane. 4. Cell Cycle Checkpoints a. There appear to be three checkpoints where the cell cycle either stops or continues onward, depending on the internal signals it receives. b. Researchers have identified a family of proteins called cyclins, internal signals that increase or decrease during the cell cycle. c. Cyclin must be present for the cell to move from the G1 stage to the S stage, and from the G2 stage to the M stage. d. The cell cycle stops at the G2 stage if DNA has not finished replicating; stopping the cell cycle at this stage allows time for repair of possible damaged DNA. e. Also, the cycle stops if chromosomes are not distributed accurately to daughter cells. f. DNA damage also stops the cycle at the G1 checkpoint by the protein p53; if the DNA is not repaired, p53 triggers apoptosis. E. Apoptosis 1. Apoptosis is programmed cell death and involves a sequence of cellular events involving: a. fragmenting of the nucleus, b. blistering of the plasma membrane, and c. engulfing of cell fragments by macrophages and/or neighboring cells. 2. Apoptosis is caused by enzymes called caspases. 3. Cells normally hold caspases in check with inhibitors. 4. Caspases are released by internal or external signals. 5. Apoptosis and cell division are balancing processes that maintain the normal level of somatic (body) cells. 6. Cell death is a normal and necessary part of development: frogs, for example, must destroy tail tissue they used as tadpoles, and the human embryo must eliminate webbing found between fingers and toes. 7. Death by apoptosis prevents a tumor from developing. Mitosis and Cytokinesis A. Eukaryotic Chromosomes 1. DNA in chromosomes of eukaryotic cells is associated with proteins; histone proteins organize chromosomes. 2. When a cell is not undergoing division, DNA in the nucleus is a tangled mass of threads called chromatin. 3. At cell division, chromatin becomes highly coiled and condensed and is now visible as individual chromosomes. 4. Each species has a characteristic number of chromosomes. a. The diploid (2n) number includes two sets of chromosomes of each type. i. The diploid number is found in all the non-sex cells of an organism's body (with a few exceptions). ii. Examples include humans (46), crayfish (200), etc. b. The haploid (n) number contains one of each kind of chromosome. i. In the life cycle of many animals, only sperm and egg cells have the haploid number. ii. Examples include humans (23), crayfish (100), etc. 5. Cell division in eukaryotes involves nuclear division and cytokinesis. a. Somatic cells undergo mitosis for development, growth, and repair. i. This nuclear division leaves the chromosome number constant. ii. A 2n nucleus replicates and divides to provide daughter nuclei that are also 2n. b. A chromosome begins cell division with two sister chromatids. i. Sister chromatids are two strands of genetically identical chromosomes. ii. At the beginning of cell division, they are attached at a centromere, a region of constriction on a chromosome. B. Stages of Mitosis 1. The centrosome, the main microtubule organizing center of the cell, divides before mitosis begins. 2. Each centrosome contains a pair of barrel-shaped organelles called centrioles. 3. The mitotic spindle contains many fibers, each composed of a bundle of microtubules. 4. Microtubules are made of the protein tubulin. a. Microtubules assemble when tubulin subunits join, disassemble when tubulin subunits become free, and form interconnected filaments of cytoskeleton. b. Microtubules disassemble as spindle fibers form. 5. Mitosis is divided into five phases: prophase, prometaphase, metaphase, anaphase, and telophase. 6. Prophase a. Nuclear division is about to occur: chromatin condenses and chromosomes become visible. b. The nucleolus disappears and the nuclear envelope fragments. c. Duplicated chromosomes are composed of two sister chromatids held together by a centromere; chromosomes have no particular orientation in the cell at this time. d. The spindle begins to assemble as pairs of centrosomes migrate away from each other. e. An array of microtubules called asters radiates toward the plasma membrane from the centrosomes. 7. Prometaphase (Late Prophase) a. Specialized protein complexes (kinetochores) develop on each side of the centromere for future chromosome orientation. b. An important event during prometaphase is attachment of the chromosomes to the spindle and their movement as they align at the metaphase plate (equator) of the spindle. c. The kinetochores of sister chromatids capture kinetochore spindle fibers. d. Chromosomes move back and forth toward alignment at the metaphase plate. 8. Metaphase a. Chromosomes, attached to kinetochore fibers, are now aligned at the metaphase plate. b. Non-attached spindle fibers, called polar spindle fibers, can reach beyond the metaphase plate and overlap. 9. Anaphase a. The two sister chromatids of each duplicated chromosome separate at the centromere. b. Daughter chromosomes, each with a centromere and single chromatid, move to opposite poles. i. Polar spindle fibers lengthen as they slide past each other. ii. Kinetochore spindle fibers disassemble at the kinetochores; this pulls daughter chromosomes to poles. iii. The motor molecules kinesin and dynein are involved in this sliding process. iv. Anaphase is the shortest stage of mitosis. 10. Telophase a. Spindle disappears in this stage. b. The nuclear envelope reforms around the daughter chromosomes. c. The daughter chromosomes diffuse, again forming chromatin. d. The nucleolus reappears in each daughter nucleus. C. Cytokinesis in Animal and Plant Cells 1. Cytokinesis in Animal Cells a. A cleavage furrow indents the plasma membrane between the two daughter nuclei at a midpoint; this deepens to divide the cytoplasm during cell division. b. Cytoplasmic cleavage begins as anaphase draws to a close and organelles are distributed. c. The cleavage furrow deepens as a band of actin filaments, called the contractile ring, constricts between the two daughter cells. d. A narrow bridge exists between daughter cells during telophase until constriction completely separates the cytoplasm. 2. Cytokinesis in Plant Cells a. The rigid cell wall that surrounds plant cells does not permit cytokinesis by furrowing. b. The Golgi apparatus produces vesicles, which move along the microtubules to a small flattened disc that has formed. c. Vesicles fuse forming a cell plate; their membranes complete the plasma membranes of the daughter cells. d. The new membrane also releases molecules from the new plant cell walls; the cell walls are strengthened by the addition of cellulose fibrils. D. The Functions of Mitosis 1. Mitosis permits growth and repair. 2. In flowering plants, the meristematic tissue retains the ability to divide throughout the life of the plant; this accounts for the continued growth, both in height and laterally, of a plant. 3. In mammals, mitosis is necessary as a fertilized egg becomes an embryo and as the embryo becomes a fetus; throughout life, mitosis allows a cut to heal or a broken bone to mend. E. Stem Cells 1. Many mammalian organs contain stem cells (or adult stem cells), which retain the ability to divide. 2. Red bone marrow stem cells repeatedly divide to produce the various types of blood cells. 3. Therapeutic cloning to produce human tissues can begin with either adult stem cells or embryonic stem cells. 4. Embryonic stem cells can be used for reproductive cloning, the production of a new individual. The Cell Cycle and Cancer (will not be on the midterm) 1. A neoplasm is an abnormal growth of cells. 2. A benign neoplasm is not cancerous; a malignant neoplasm is cancerous. 3. Cancer is a cellular growth disorder that results from the mutation of genes that regulate the cell cycle; i.e., cancer results from the loss of control and a disruption of the cell cycle. 4. Carcinogenesis, the development of cancer is gradual—it may take decades before a cell has the characteristics of a cancer cell. B. Characteristics of Cancer Cells 1. Cancer cells lack differentiation. a. Unlike normal cells that differentiate into muscle or nerves cells, cancer cells have an abnormal form and are nonspecialized. b. Normal cells enter the cell cycle only about 50 times; cancer cells are immortal in that they can enter the cell cycle repeatedly. 2. Cancer cells have abnormal nuclei. a. The nuclei may be enlarged and may have an abnormal number of chromosomes. b. The chromosomes have mutated; some chromosomes may be duplicated or deleted. c. Gene amplification, extra copies of genes, is more frequent in cancerous cells. d. Whereas ordinary cells with DNA damage undergo apoptosis, cancer cells do not. 3. Cancer cells form tumors. a. Normal cells are anchored and stop dividing when in contact with other cells; i.e., they exhibit contact inhibition. b. Cancer cells invade and destroy normal tissue and their growth is not inhibited. c. Cancer cells pile on top of each other to form a tumor. 4. Cancer cells undergo metastasis and angiogenesis. a. A benign tumor is encapsulated and does not invade adjacent tissue. b. Cancer in situ is a tumor in its place of origin but is not encapsulated—it will invade surrounding tissues. c. Many types of cancer can undergo metastasis, in which new tumors form which are distant from the primary tumor. d. Angiogenesis, the formation of new blood vessels, is required to bring nutrients and oxygen to the tumor. C. D. E. F. e. A cancer patient's prognosis depends on whether the tumor has invaded surrounding tissue, whether there is lymph node involvement, and whether there are metastatic tumors elsewhere in the body. Origin of Cancer 1. A DNA repair system corrects mutations during replication; mutations in genes encoding the various repair enzymes can cause cancer. 2. Proto-oncogenes specify proteins that stimulate the cell cycle while tumor-suppressor genes specify proteins that inhibit the cell cycle; mutations of either of these genes can cause cancer. 3. DNA segments called telomeres form the ends of chromosomes and shorten with each replication, eventually signaling the cell to end division; cancer cells produce telomerase that keeps telomeres at a constant length and thus the cells to continue dividing. Regulation of the Cell Cycle 1. Proto-oncogenes are at the end of a stimulatory pathway from the plasma membrane to the nucleus; a growth factor binding at the plasma membrane can result in turning on an oncogene. 2. Tumor-suppressor genes are at the end of an inhibitory pathway; a growth-inhibitory factor can result in turning on a tumor suppressor gene that inhibits the cell cycle. 3. The balance between stimulatory and inhibitory signals determines whether proto-oncogenes or tumor-suppressor genes are active, and therefore whether or not cell division occurs. Oncogenes 1. Proto-oncogenes can undergo mutation to become oncogenes (cancer-causing genes). 2. An oncogene may code for a faulty receptor in the stimulatory pathway, or, 3. An oncogene can specify an abnormal protein product or abnormally high levels of a normal product that stimulates the cell cycle. 4. About 100 oncogenes have been described; the ras gene family includes variants associated with lung, colon, pancreatic cancers as well as leukemias, lymphomas, and thyroid cancers; the BRCA1gene is associated with certain forms of breast and ovarian cancer. Tumor-suppressor Genes 1. Mutation of a tumor-suppressor gene results in unregulated cell growth. 2. Researchers have identified about a half dozen tumor-suppressor genes. 3. The RB tumor-suppressor gene prevents retinoblastoma, a cancer of the retina, and has been found to malfunction in cancers of the breast, prostate, bladder, and small-cell lung carcinoma. 4. The p53 tumor-suppressor gene is more frequently mutated in human cancers than any other known gene; it normally functions to trigger cell cycle inhibitors and stimulate apoptosis. Prokaryotic Cell Division 1. Unicellular organisms reproduce via asexual reproduction, in which the offspring are genetically identical to the parent. B. The Prokaryotic Chromosome 1. Prokaryotic cells (bacteria and archaea) lack a nucleus and other membranous organelles. 2. The prokaryotic chromosome is composed of DNA and associated proteins, but much less protein than eukaryotic chromosomes. 3. The chromosome appears as a nucleoid, an irregular-shaped region that is not enclosed by a membrane. 4. The chromosome is a circular loop attached to the inside of the plasma membrane; it is about 1,000 times the length of the cell. C. Binary Fission 1. Binary fission of prokaryotic cells produces two genetically identical daughter cells. 2. Before cell division, DNA is replicated--both chromosomes are attached to a special site inside the plasma membrane. 3. The two chromosomes separate as a cell lengthens and pulls them apart. 4. When the cell is approximately twice its original length, the plasma membrane grows inward, a septum (consisting of new cell wall and plasma membrane) forms, dividing the cell into two daughter cells. 5. The generation time of bacteria depends on the species and environmental conditions; Escherichia coli's generation time is about 20 minutes. D. Comparing Prokaryotes and Eukaryotes 1. Both binary fission and mitosis ensure that each daughter cell is genetically identical to the parent. 2. Bacteria and protists use asexual reproduction to produce identical offspring. 3. In multicellular fungi, plants, and animals, cell division is part of the growth process that produces and repairs the organism. 4. Prokaryotes have a single chromosome with mostly DNA and some associated protein; there is no spindle apparatus. 5. Eukaryotic cells have chromosomes with DNA and many associated proteins; histone proteins organize the chromosome. 6. The spindle is involved in distributing the daughter chromosomes to the daughter nuclei. Meiosis - be sure to watch the bio flicks of meiosis in there is a lovely comparison of mitosis and meiosis on the MP3 tutor. Remember the diagrams replace notes in that a lot of the detail in these notes is replaced by your ability to recognize structures and their movement within diagrams. 13 Halving the Chromosome Number 1. Meiosis is nuclear division, reducing the chromosome number from the diploid (2n) to the haploid (n) number. 2. The haploid (n) number is half of the diploid number of chromosomes. 3. Sexual reproduction requires gamete (reproductive cell, often sperm and egg) formation and then fusion of gametes to form a zygote. 4. A zygote always has the full or diploid (2n) number of chromosomes. 5. If gametes contained same number of chromosomes as body cells, doubling would soon fill cells. B. Homologous Pairs of Chromosomes 1. In diploid body cells, chromosomes occur as pairs. a. Each set of chromosomes is a homologous pair; each member is a homologous chromosome or homologue. b. Homologues look alike, have the same length and centromere position, and have a similar banding pattern when stained. c. A location on one homologue contains gene for the same trait that occurs at this locus on the other homologue, although the genes may code for different variations of that trait; alternate forms of a gene are called alleles. 2. Chromosomes duplicate immediately prior to nuclear division. a. Duplication produces two identical parts called sister chromatids; they are held together at the centromere. 3. One member of each homologous pair is inherited from the male parent, the other member from the female parent. 4. One member of each homologous pair will be placed in each sperm or egg. C. Overview of Meiosis 1. Meiosis involves two nuclear divisions and produces four haploid daughter cells. 2. Each daughter cell has half the number of chromosomes found in the diploid parent nucleus. 3. Meiosis I is the nuclear division at the first meiotic division. a. Prior to meiosis I, DNA replication occurs, each chromosome thus has two sister chromatids. b. During meiosis I, homologous chromosomes pair; this is called synapsis. c. During synapsis, the two sets of paired chromosomes lay alongside each other as a bivalent (sometimes called a tetrad). 4. In meiosis II, the centromeres divide and daughter chromosomes (derived as sister chromatids) separate. a. No replication of DNA is needed between meiosis I and II because chromosomes are already doubled (DNA replication occurred prior to meiosis I). b. Chromosomes in the four daughter cells have only one chromatid. c. Counting the number of centromeres verifies that parent cells were diploid; each daughter cell is haploid. d. In the animal life cycle, daughter cells become gametes that fuse during fertilization. e. Fertilization restores the diploid number in cells. Genetic Variation A. Genetic Recombination 1. Due to genetic recombination, offspring have a different combination of genes than their parents. 2. Without recombination, asexual organisms must rely on mutations to generate variation among offspring; this is sufficient because they have great numbers of offspring. 3. Meiosis brings about genetic recombination in two ways: crossing-over and independent assortment. 4. Crossing-over of non-sister chromatids results in exchange of genetic material between nonsister chromatids of a bivalent; this introduces variation. 5. At synapsis, homologous chromosomes are held in position by a nucleoprotein lattice (the synaptonemal complex). 6. As the lattice of the synaptonemal complex breaks down at the beginning of anaphase I, homologues are temporarily held together by chiasmata, regions where the non-sister chromatids are attached due to crossing-over. 7. The homologues separate and are distributed to daughter cells. 8. Due to this genetic recombination, daughter chromosomes derived from sister chromatids are no longer identical. B. Independent Assortment of Homologous Chromosomes 1. During independent assortment, the homologous chromosomes separate independently or in a random manner. 2. Independent assortment in a cell with only three pairs of chromosomes is 23 or eight combinations of maternal and paternal chromosomes. 3. In humans with 23 pairs of chromosomes, the combinations possible are 223 or 8,388,608, and this does not consider the variation from crossing-over. C. Fertilization 1. When gametes fuse at fertilization, chromosomes donated by parents combine. 2. The chromosomally different zygotes from same parents have (223)2 or 70,368,744,000,000 combinations possible without crossing-over. 3. If crossing-over occurs once, then (423)2 or 4,951,760,200,000,000,000,000,000,000 genetically different zygotes are possible for one couple. D. Significance of Genetic Recombination 1. A successful parent in a particular environment can reproduce asexually and produce offspring adapted to that environment. 2. If the environment changes, differences among offspring provide the sexual parents with much improved chances of survival. The Phases of Meiosis 1. Both meiosis I and meiosis II have four phases: prophase, metaphase anaphase, and telophase. B. Prophase I 1. Nuclear division is about to occur: nucleolus disappears; nuclear envelope fragments; centrosomes migrate away from each other; and spindle fibers assemble. 2. Homologous chromosomes undergo synapsis to form bivalents; crossing-over may occur at this time in which case sister chromatids are no longer identical. 3. Chromatin condenses and chromosomes become microscopically visible. C. Metaphase I 1. Bivalents held together by chiasmata have moved toward the metaphase plate at the equator of the spindle. 2. In metaphase I, there is a fully formed spindle and alignment of the bivalents at the metaphase plate. 3. Kinetochores, protein complexes just outside the centromeres attach to spindle fibers called kinetochore spindle fibers. 4. Bivalents independently align themselves at the metaphase plate of the spindle. 5. Maternal and paternal homologues of each bivalent may be oriented toward either pole. D. Anaphase I 1. The homologues of each bivalent separate and move toward opposite poles. 2. Each chromosome still has two chromatids. E. Telophase I 1. In animals, this stage occurs at the end of meiosis I. 2. When it occurs, the nuclear envelope reforms and nucleoli reappear. 3. This phase may or may not be accompanied by cytokinesis. F. Interkinesis 1. This period between meiosis I and II is similar to the interphase between mitotic divisions; however, no DNA replication occurs (the chromosomes are already duplicated). G. Meiosis II 1. During metaphase II, the haploid number of chromosomes align at the metaphase plate. 2. During anaphase II, the sister chromatids separate at the centromeres; the two daughter chromosomes move toward the poles. 3. Due to crossing-over, each gamete can contain chromosomes with different types of genes. 4. At the end of telophase II and cytokinesis, there are four haploid cells. 5. In animals, the haploid cells mature and develop into gametes. 6. In plants, the daughter cells become spores and divide to produce a haploid generation; these haploid cells fuse to become a zygote that develops into a diploid generation. 7. The type of life cycle of alternating haploid and diploid generations is called alternation of generations. Meiosis Compared to Mitosis 1. Meiosis requires two nuclear divisions; mitosis requires only one nuclear division. 2. Meiosis produces four daughter nuclei and four daughter cells; mitosis produces only two. 3. The daughter cells produced by meiosis are haploid; the daughter cells produced by mitosis are diploid. 4. The daughter cells produced by meiosis are not genetically identical; the daughter cells produced by mitosis are genetically identical to each other and to the parental cell. B. A Occurrence 1. In humans, meiosis occurs only in reproductive organs to produce gametes. 2. Mitosis occurs in all tissues for growth and repair. C. Meiosis I Compared to Mitosis 1. DNA is replicated only once before both mitosis and meiosis; in mitosis there is only one nuclear division; in meiosis there are two nuclear divisions. 2. During prophase I of meiosis, homologous chromosomes pair and undergo crossing-over; this does not occur during mitosis. 3. During metaphase I of meiosis, bivalents align at the metaphase plate; in mitosis individual chromosomes align. 4. During anaphase I in meiosis, homologous chromosomes (with centromeres intact) separate and move to opposite poles; in mitosis at this stage, sister chromatids separate and move to opposite poles. D. Meiosis II Compared to Mitosis 1. Events of meiosis II are the same stages as in mitosis. 2. However, in meiosis II, the nuclei contain the haploid number of chromosomes. Human Life Cycle 1. Life cycle refers to all reproductive events between one generation and next. 2. In animals, the adult is always diploid [Instructors note: some bees, etc. have haploid male adults]. 3. In plants, there are two adult stages: one is diploid (called the sporophyte) and one is haploid (called the gametophyte). 4. Mosses are haploid most of their cycle; the majority of higher plants are diploid most of their cycle. 5. In fungi and some algae, only the zygote is diploid, and it undergoes meiosis. 6. In human males, meiosis is part of spermatogenesis (the production of sperm), and occurs in the testes. 7. In human females, meiosis is part of oogenesis (the production of eggs), and occurs in the ovaries. 8. After birth, mitotic cell division is involved in growth and tissue regeneration of somatic tissue. B. Spermatogenesis and Oogenesis in Humans 1. Spermatogenesis a. In the testes of males, primary spermatocytes with 46 chromosomes undergo meiosis I to form two secondary spermatocytes, each with 23 duplicated chromosomes. b. Secondary spermatocytes divide (meiosis II) to produce four spermatids, also with 23 daughter chromosomes. c. Spermatids then differentiate into sperm (spermatozoa). d. Meiotic cell division in males always results in four cells that become sperm. 2. Oogenesis a. In the ovaries of human females, primary oocytes with 46 chromosomes undergo meiosis I to form two cells, each with 23 duplicated chromosomes. b. One of the cells, a secondary oocyte, receives almost all the cytoplasm; the other cell, a polar body, disintegrates or divides again. c. The secondary oocyte begins meiosis II and then stops at metaphase II. d. At ovulation, the secondary oocyte leaves the ovary and enters an oviduct where it may meet a sperm. e. If a sperm enters secondary oocyte, the oocyte is activated to continue meiosis II to completion; the result is a mature egg and another polar body, each with 23 daughter chromosomes. f. Meiosis produces one egg and three polar bodies; polar bodies serve to discard unnecessary chromosomes and retain most of the cytoplasm in the egg. g. The cytoplasm serves as a source of nutrients for the developing embryo. I consider the beginning of meiosis the start of the genetics unit as it sets in place the basis of sexual reproduction and gametes. Without question you need to practice your crosses to get the basics of Mendelian genetics. Make sure you review the chromosomal basis of inheritance MP3 tutor and the activities on Mono hybrid and dihybrid crosses. Remember that genes are on chromosomes in the Mendel's ideas were not exactly correct. Also recall that the basic discovery of genes being on chromosomes was accomplished by Sutton in his lab and his famous graduate student was Morgan who discovered sex linkage in fruit flies. 14 Gregor Mendel - the history is nice but it's more important to understand how to do crosses. 1. 2. 3. 4. 5. Mendel was an Austrian monk. Mendel formulated two fundamental laws of heredity in the early 1860s. He had previously studied science and mathematics at the University of Vienna. At time of his research, he was a substitute science teacher at a local technical high school. Prior to Mendel's work, investigators had been trying to support a "blending" concept of inheritance. B. Blending Concept of Inheritance 1. This theory stated that offspring would have traits intermediate between those of the parents. 2. Red and white flowers produce pink flowers; any return to red or white offspring was considered instability in the genetic material. 3. Charles Darwin wanted to develop a theory of evolution based on hereditary principles; blending theory was of no help. a. A blending theory did not account for variation (differences) and could not explain species diversity. b. The particulate theory of inheritance proposed by Mendel can account for presence of differences among members of a population generation after generation. c. Mendel's work was unrecognized until 1900; Darwin was never able to use it to support his theory of evolution. C. Mendel's Experimental Procedure 1. Because Mendel had a mathematical background, he used a statistical basis for his breeding experiments. 2. Mendel prepared his experiments carefully and conducted preliminary studies. a. He chose the garden pea, Pisum sativum, because peas were easy to cultivate, had a short generation time, and could be cross-pollinated by hand. b. From many varieties, Mendel chose 22 true-breeding varieties for his experiments. c. True-breeding varieties had all offspring like the parents and like each other. d. Mendel studied simple traits (e.g., seed shape and color, flower color, etc.). 3. Mendel traced inheritance of individual traits and kept careful records of numbers. 4. He used his understanding of mathematical principles of probability to interpret results. 5. He arrived at a particulate theory of inheritance because it is based on the existence of minute particles—now called genes. Mendel's Law of Segregation 1. Mendel confirmed that his tall plants always had tall offspring, i.e., were true-breeding, before crossing two different strains that differed in only one trait—this is called a monohybrid cross. 2. A monohybrid cross is between two parent organisms true-breeding for two distinct forms of one trait. 3. Mendel tracked each trait through two generations. a. P generation is the parental generation in a breeding experiment. b. F1 generation is the first-generation offspring in a breeding experiment. c. F2 generation is the second-generation offspring in a breeding experiment. 4. He performed reciprocal crosses, i.e. pollen of tall plant to stigma of short plant and viceversa. 5. His results were contrary to those predicted by a blending theory of inheritance. B. C. D. E. 6. He found that the F1 plants resembled only one of the parents. 7. Characteristics of other parent reappeared in about 1/4 of F2 plants; 3/4 of offspring resembled the F1 plants. 8. Mendel saw that these 3:1 results were possible if: a. F1 hybrids contained two factors for each trait, one being dominant and the other recessive; b. factors separated when gametes were formed; a gamete carried one copy of each factor; c. and random fusion of all possible gametes occurred upon fertilization. 9. Results of his experiments led Mendel to develop his first law of inheritance—the law of segregation: a. Each organism contains two factors for each trait. b. Factors segregate in the formation of gametes. c. Each gamete contains one factor for each trait. d. Fertilization gives each new individual two factors for each trait. As Viewed by Modern Genetics 1. Each trait in a pea plant is controlled by two alleles, alternate forms of a gene that occur at the same gene locus on homologous chromosomes. 2. A dominant allele masks or hides expression of a recessive allele; it is represented by an uppercase letter. 3. A recessive allele is an allele that exerts its effect only in the homozygous state; its expression is masked by a dominant allele; it is represented by a lowercase letter. 4. The gene locus is the specific location of alleles on homologous chromosomes. 5. The process of meiosis explains Mendel's law of segregation. 6. In Mendel's cross, the parents were true-breeding; each parent had two identical alleles for a trait–they were homozygous, indicating they possess two identical alleles for a trait. 7. Homozygous dominant genotypes possess two dominant alleles for a trait. 8. Homozygous recessive genotypes possess two recessive alleles for a trait. 9. After cross-pollination, all individuals of the F1 generation had one of each type of allele. 10. Heterozygous genotypes possess one of each allele for a particular trait. 11. The allele not expressed in a heterozygote is a recessive allele. Genotype Versus Phenotype 1. Two organisms with different allele combinations can have the same outward appearance (e.g., TT and Tt pea plants are both tall; therefore, it is necessary to distinguish between alleles present and the appearance of the organism). 2. Genotype refers to the alleles an individual receives at fertilization (dominant, recessive). 3. Phenotype refers to the physical appearance of the individual (tall, short, etc.). One-trait Genetics Problems 1. First determine which characteristic is dominant; then code the alleles involved. 2. Determine the genotype and gametes for both parents; an individual has two alleles for each trait; each gamete has only one allele for each trait. 3. Each gamete is haploid; each has a 50% chance of receiving either allele. Laws of Probability 1. Probability is the likely outcome a given event will occur from random chance. a. For example, with every coin flip there is a 50% chance of heads and 50% chance of tails. b. Chance of inheriting one of either two alleles from a parent is also 50%. 2. The multiplicative law of probability states that the chance of two or more independent events occurring together is the product of the probability of the events occurring separately. a. The chance of inheriting a specific allele from one parent and a specific allele from another is ½ x ½ or 1/4. b. Possible combinations for the alleles Ee of heterozygous parents are the following: EE = ½ x ½ = 1/4 eE = ½ x ½ = 1/4 Ee = ½ x ½ = 1/4 ee = ½ x ½ = 1/4 3. The additive law of probability calculates the probability of an event that occurs in two or more independent ways; it is the sum of individual probabilities of each way an event can occur; in the above example where unattached earlobes are dominant (EE,Ee, and eE), the chance for unattached earlobes is 1/4 + 1/4 + 1/4 = 3/4. F. The Punnett Square 1. The Punnett square was introduced by R. C. Punnett (early 1900s) and provides a simple method to calculate the probable results of a genetic cross. 2. In a Punnett square, all possible types of sperm alleles are lined up vertically and all possible egg alleles are lined up horizontally; every possible combination is placed in squares. 3. The larger the sample size examined, the more likely the outcome will reflect predicted ratios; a large number of offspring must be counted to observe the expected results; only in that way can all possible genetic types of sperm fertilize all possible types of eggs. 4. Specific crosses in humans cannot be done in order to count many offspring; therefore in humans, the phenotypic ratio is used to estimate the probability of any child having a particular characteristic. 5. Punnett square uses laws of probability; it does not dictate what the next child will inherit. 6. "Chance has no memory": if two heterozygous parents have a first child with attached earlobes (likely in 1/4th of children), a second child born still has 1/4 chance of having attached earlobes. G. One-Trait Testcross 1. To confirm that the F1 was heterozygous, Mendel crossed his F1 plants with homozygous recessive plants. 2. Results indicated the recessive factor was present in the F1 plants; they were thus heterozygous. 3. A monohybrid testcross is used between an individual with dominant phenotype and an individual with a recessive phenotype to see if the individual with dominant phenotype is homozygous or heterozygous. Mendel's Law of Independent Assortment 1. This two-trait (dihybrid) cross is between two parent organisms that are true-breeding for different forms of two traits; it produces offspring heterozygous for both traits. 2. Mendel observed that the F1 individuals were dominant in both traits. 3. 3.. He further noted four phenotypes among F2 offspring; he deduced second law of heredity. 4. Mendel's law of independent assortment states that members of one pair of factors assort independently of members of another pair, and that all combinations of factors occur in gametes. B. Two-trait Genetics Problems 1. Laws of probability indicate a 9:3:3:1 phenotypic ratio of F2 offspring resulting in the following: a. 9/16 of the offspring are dominant for both traits; b. 3/16 of the offspring are dominant for one trait and recessive for the other trait; c. 3/16 of the offspring are dominant and recessive opposite of the previous proportions; and d. 1/16 of the offspring are recessive for both traits. 2. The Punnett Square for two-trait crosses a. A larger Punnett square is used to calculate probable results of this cross. b. A phenotypic ratio of 9:3:3:1 is expected when heterozygotes for two traits are crossed and simple dominance is present for both genes. c. Independent assortment during meiosis explains these results. C. Two-Trait Testcross 1. A two-trait testcross tests if individuals showing two dominant characteristics are homozygous for both or for one trait only, or heterozygous for both. 2. If an organism heterozygous for two traits is crossed with another recessive for both traits, the expected phenotypic ratio is 1:1:1:1. 3. In dihybrid genetics problems, the individual has four alleles, two for each trait. Human Genetic Disorders - it is important to understand pathology, this helps us understand what is normal. You're going to need to know some genetic diseases as examples of how they are transmitted within species such as humans. A. Patterns of Inheritance 1. Genetic disorders are medical conditions caused by alleles inherited from parents. 2. An autosome is any chromosome other than a sex (X or Y) chromosome. 3. In a pedigree chart, males are designated by squares, females by circles; shaded circles and squares are affected individuals; line between square and circle represents a union; vertical line leads to offspring. 4. A carrier is a heterozygous individual with no apparent abnormality but able to pass on an allele for a recessively-inherited genetic disorder. 5. Autosomal dominant and autosomal recessive alleles have different patterns of inheritance. a. Characteristics of autosomal dominant disorders i. Affected children usually have an affected parent. ii. Heterozygotes are affected.: two affected parents can produce unaffected child; two unaffected parents will not have affected children. b. Characteristics of autosomal recessive disorders i. Most affected children have normal parents since heterozygotes have a normal phenotype. ii. Two affected parents always produce an affected child. iii. Close relatives who reproduce together are more likely to have affected children. B. Autosomal Recessive Disorders 1. Tay-Sachs Disease a. Usually occurs among Jewish people in the U.S. of central and eastern European descent. b. Symptoms are not initially apparent; infant's development begins to slow between four to eight months, neurological and psychomotor difficulties become apparent, child gradually becomes blind and helpless, develops seizures, eventually becomes paralyzed and dies by age of three or four. c. This results from lack of enzyme hexosaminidase A (Hex A) and the subsequent storage of its substrate, glycosphingolipid, in lysosomes. d. Primary sites of storage are cells of the brain; accounts for progressive deterioration. e. There is no treatment or cure. f. Prenatal diagnosis is possible by amniocentesis or chorionic villi sampling. g. The gene is located on chromosome 15. 2. Cystic Fibrosis a. This is the most common lethal genetic disease in Caucasians in the U.S. b. About 1 in 20 Caucasians is a carrier, and about 1 in 3,000 newborns has this disorder. c. An increased production of a viscous form of mucus in the lungs and pancreatic ducts is seen. i. The resultant accumulation of mucus in the respiratory tract interferes with gas exchange. ii. Digestive enzymes must be mixed with food to supplant the pancreatic juices. d. New treatments have raised the average life expectancy to up to 35 years. e. Chloride ions (Cl–) fail to pass plasma membrane proteins. f. Since water normally follows Cl–, lack of water in the lungs causes thick mucus. g. The cause is a gene on chromosome 7; attempts to insert the gene into nasal epithelium has had little success. h. Genetic testing for adult carriers and fetuses is possible. 3. Phenylketonuria (PKU) a. PKU occurs once in every 5,000 births; it is the most common inherited disease of the nervous system. b. It is caused by a lack of an enzyme needed to metabolize amino acid phenylalanine; this results in accumulation of the amino acid in nerve cells of the brain and impairs nervous system development. c. PKU is caused by a gene on chromosome 12. d. Newborns are routinely tested in the hospital for high levels of phenylalanine in the blood. e. If an infant has PKU, the child is placed on a diet low in phenylalanine until the brain is fully developed, near age seven. 4. Sickle-Cell Disease a. This disease is the most common inherited disorder in blacks, affecting about 1 in 500 African Americans. b. The gene is on chromosome 11. c. In affected individuals, the red blood cells are shaped like sickles—an abnormal hemoglobin molecule, Hbs, causes the defect. i. Normal hemoglobin, HbA, differs from Hbs by one amino acid in the protein globin. d. The disease is an example of pleiotropy, describing a gene that affects more than one characteristic of an individual. e. Sickling of the red blood cells occurs when the oxygen content of the person's blood is low, thereby slowing down blood flow and clogging small vessels. f. Signs and symptoms include anemia, weakness, fever, pain, rheumatism, low resistance to disease, kidney and heart failure. g. Treatment includes pain management, blood transfusions, and bone marrow transplants. h. The disease can be diagnosed prenatally. i. Individuals with the sickle cell trait (carriers), who normally do not have any sickleshaped cells unless they experience dehydration or mild oxygen deprivation, are resistant to the disease malaria. C. Autosomal Dominant Disorders 1. Neurofibromatosis a. This is an autosomal dominant disorder that affects one in 3,500 newborns and is distributed equally around the world. b. Affected individuals have tan skin spots at birth, which develop into benign tumors. c. Neurofibromas are lumps under the skin comprised of fibrous coverings of nerves. d. In most cases, symptoms are mild and patients live a normal life; sometimes symptoms are severe: i. skeletal deformities, including a large head; ii. eye and ear tumors that can lead to blindness and hearing loss; and iii. learning disabilities and hyperactivity. iv. Such variation is called variable expressivity. e. The gene that codes for neurofibromatosis was discovered in 1990 to be on chromosome 17. i. The gene controls production of neurofibromin protein that normally blocks growth signals for cell division. ii. Many types of mutations result in this effect. iii. Some mutations are caused by a gene that moves from another location in the genome. 2. Huntington Disease a. This leads to progressive degeneration of brain cells, which in turn causes severe muscle spasm, personality disorders, and death in 10–15 years after onset. b. Most appear normal until they are of middle age and already have had children who might carry the gene; occasionally, first signs of the disease are seen in teenagers or even younger. c. The gene for Huntington disease is located on chromosome 4. d. This gene contains many repeats of a base triplet that codes for glutamine in the huntingtin protein; normal persons have 10–15 glutamines; affected persons have 36 or more. e. A huntingtin protein with over 36 glutamines changes shape and forms large clumps inside neurons; it also attracts other proteins to clump with it. 3. Achondroplasia a. This disease is a common form of dwarfism, associated with a defect in the growth of long bones. b. Affected individuals have short arms and legs, a sway back, and a normal torso and head. c. About 1 in 25,000 people have the disease. d. Individuals with the disease are heterozygotes (Aa); the homozygous recessive (aa) condition yields normal-length limbs, while the homozygous dominant (AA) condition is lethal. Beyond Mendelian Genetics A. Incomplete Dominance 1. Incomplete dominance: offspring show traits intermediate between two parental phenotypes. a. True-breeding red and white-flowered four-o'clocks produce pink-flowered offspring. b. Incomplete dominance has a biochemical basis; the level of gene-directed protein production may be between that of the two homozygotes. c. One allele of a heterozygous pair only partially dominates expression of its partner. d. This does not support a blending theory; parental phenotypes reappear in F2 generation. B. Human Examples of Incomplete Dominance 1. Curly versus Straight Hair 2. A curly-haired Caucasian and a straight-haired Caucasian will have wavy-haired offspring. 3. Two wavy-haired parents will produce a 1:2:1 ratio of curly-wavy-straight hair children. 4. Sickle-cell disease, Tay Sachs disease, and cystic fibrosis are considered examples of incomplete dominance. C. Multiple Allelic Traits 1. This occurs when a gene has many allelic forms or alternative expressions. 2. ABO Blood Types a. The ABO system of human blood types is a multiple allele system. b. Two dominant alleles (IAand IB) code for presence of A and B glycoproteins on red blood cells. c. This also includes a recessive allele (i°) coding for no A or B glycoproteins on red blood cells. d. As a result, there are four possible phenotypes (blood types): A, B, AB, and O e. This is a case of codominance, where both alleles are fully expressed. 3. The Rh factor is inherited independently from the ABO system; the Rh+ allele is dominant. D. Polygenic Inheritance 1. Polygenic inheritance occurs when one trait is governed by two or more sets of alleles. 2. Dominant alleles have a quantitative effect on the phenotype: each adds to the effect. 3. The more genes involved, the more continuous is the variation in phenotypes, resulting in a bellshaped curve. 4. Crosses of white and dark-red wheat seeds produce seeds with seven degrees of intermediate colors due to genes at three separate loci. 5. Human Examples of Polygenic Inheritance a. A hybrid cross for skin color provides a range of intermediates. b. Parents with intermediate skin color can produce children with the full range of skin colors. c. Albinism, where one gene interferes with the expression of others, is an example of epistasis. E. Polygenic Disorders 1. This includes cleft lip, clubfoot, congenital dislocations of the hip, hypertension, diabetes, schizophrenia, allergies and cancers. 2. Behavioral traits including suicide, phobias, alcoholism, and homosexuality may be associated with particular genes but are not likely completely predetermined. 3. Environment and the Phenotype a. In water buttercups, the aquatic environment dramatically influences the structure of the plant. b. Temperature triggers a primrose to develop white flowers when grown above 32°C and red flowers when grown at 24°C. c. The coats of Siamese cats and Himalayan rabbits have darker tipped ears, nose, paws, etc. due to the enzyme encoded by an allele which is only active at the extremities at low temperatures. F. Environment and the Phenotype 1. Both genotype and the environment affect the phenotype. 2. Water and temperature can have profound influence on the phenotype. a. A flower might be one color at one temperature and another color at another temperature. b. The coat color of certain animals can change with temperature. 15 Chromosomal Inheritance 1. Genes are located on chromosomes; this is called the chromosome theory of inheritance. 2. Chromosomes can be categorized as two types: a. Autosomes are non-sex chromosomes that are the same number and kind between sexes. b. Sex chromosomes determine if the individual is male or female. 3. Sex chromosomes in the human female are XX; those of the male are XY. 4. Males produce X-containing and Y-containing gametes; therefore males determine the sex of offspring. 5. Besides genes that determine sex, sex chromosomes carry many genes for traits unrelated to sex. 6. An X-linked gene is any gene located on X chromosome; used to describe genes on X chromosome that are missing on the Y chromosome. B. X-Linked Alleles 1. Work with fruit flies (Drosophila) by Thomas Hunt Morgan (early 1900s) confirmed genes were on chromosomes. a. Fruit flies are easily and inexpensively raised in common laboratory glassware. b. Females only mate once and lay hundreds of eggs. c. The fruit fly generation time is short, allowing rapid experiments. 2. Fruit flies have an XY sex chromosome system similar to the human system; experiments can be correlated to the human situation. a. Newly discovered mutant male fruit flies had white eyes. b. Cross of the hybrids from the white-eyed male crossed with a dominant red-eyed female yielded the expected 3:1 red-to-white ratio; however, all of the white-eyed flies were males. c. An allele for eye color on the X but not on the Y chromosome supports the results of this cross. d. Behavior of this allele corresponds to the behavior of the chromosome; this confirmed the chromosomal theory of inheritance. 3. X-Linked Problems a. X-linked alleles are designated as superscripts to the X chromosome. b. Heterozygous females are carriers; they do not show the trait but can transmit it. c. Males are never carriers but express the one allele on the X chromosome; the allele could be dominant or recessive. d. One form of color-blindness is X-linked recessive. C. Human X-Linked Disorders 1. More males have X-linked traits because recessive alleles on the X chromosome in males are expressed in males. 2. Color Blindness a. Color blindness can be an X-linked recessive disorder involving mutations of genes coding for green or red sensitive cone cells, resulting in the inability to perceive green or red, respectively; the pigment for blue-sensitive protein is autosomal. b. About 8% of Caucasian men have red-green color blindness. 3. Muscular Dystrophy a. Duchenne muscular dystrophy is the most common form and is characterized by wasting away of muscles, eventually leading to death; it affects one out of every 3,600 male births. b. This X-linked recessive disease involves a mutant gene that fails to produce the protein dystrophin. c. Signs and symptoms (e.g., waddling gait, toe walking, frequent falls, difficulty in rising) soon appear. d. Muscles weaken until the individual is confined to a wheelchair; death usually occurs by age 20. e. Affected males are rarely fathers; the gene passes from carrier mother to carrier daughter. f. Lack of dystrophin protein causes calcium ions to leak into muscle cells; this promotes action of an enzyme that dissolves muscle fibers. g. As the body attempts to repair tissue, fibrous tissue forms and cuts off blood supply to the affected muscles. h. A test now detects carriers of Duchenne muscular dystrophy; treatments are being attempted. 4. Hemophilia a. About one in 10,000 males is a hemophiliac with impaired ability of blood to clot. b. The two common types: Hemophilia A, due to the absence of clotting factor IX; Hemophilia B, due to the absence of clotting factor VIII. c. Hemophiliacs bleed externally after an injury and also suffer internal bleeding around joints. d. Hemorrhages stop with transfusions of blood (or plasma) or concentrates of clotting protein. e. Factor VIII is now available as a genetically-engineered product. f. Of Queen Victoria's 26 offspring, five grandsons had hemophilia and four granddaughters were carriers. 5. Fragile X Syndrome (See Health and Focus box) a. In this case, the X chromosome is nearly broken; most often found in males. b. This affects one in 1,500 males and one in 2,500 females. c. As children, they are often hyperactive or autistic with delayed or repetitive speech. d. As adults, males usually have larger testes, unusually protruding ears, and other symptoms. e. About one-fifth of males with fragile X do not show symptoms. f. Fragile X passes from a symptomless male carrier to grandson. g. It has been traced to excessive repeats of base triplet CGG (cytosine-guanine-guanine); up to 230 copies compared to normal 6–to–50 copies. Gene Linkage 1. Fruit flies have four pairs of chromosomes to hold thousands of genes; therefore each chromosome must hold many genes. 2. All alleles on one chromosome form a linkage group that are inherited together except when crossing over occurs. 3. Crossing-over causes recombinant gametes and at fertilization, recombinant phenotypes. 4. Linked alleles do not obey Mendel's laws because they tend to go into the gametes together. 5. A linkage map (or chromosome map) tells the relative distances between gene loci on a chromosome. B. Constructing a Chromosome Map 1. The percentage of recombinant phenotypes, which is statistically related to the frequency of crossing over between specific loci, is used to measure the distance between genes. 2. Crosses involving linked genes do not give the same results as unlinked genes. 3. A heterozygote forms only two types of gametes and produces offspring with only two phenotypes. C. Linkage Data 1. Linked genes indicate the distance between genes on the chromosomes. 2. If 1% of crossing-over equals one map unit, then 6% recombinants reveal 6 map units between genes. 3. If crosses are performed for three alleles on a chromosome, only one map order explains map units. 4. Humans have few offspring and a long generation time, and it is impossible to designate matings; therefore biochemical methods are used to map human chromosomes. Changes in Chromosome Numbers 1. Chromosomal mutations are changes in chromosome number or structure. 2. Mutations, along with crossing-over, recombination of chromosomes during meiosis, and gamete fusion during fertilization, increase the amount of variation among offspring. 3. The correct number of chromosomes in a species is called euploidy; changes in chromosome number include polyploidy and aneuploidy. B. Polyploidy 1. A polyploid is a eukaryote with three or more complete sets of chromosomes. 2. Polyploid organisms are named according to the number of sets of chromosomes they have: triploids (3n), tetraploids (4n), etc. 3. Polyploidy is not often seen in animals. 4. Polyploidy is a major evolutionary mechanism in plants; it is probably involved in 47% of flowering plants including some important crops (wheat, corn, fruits, etc.). 5. Polyploidy generally arises following hybridization (reproduction between two different species); a hybrid may have an odd number of chromosomes and thus be sterile, but if the chromosomes in the hybrid double in number, the now-even number of chromosomes can undergo synapsis during meiosis; successful polyploidy thus results in a new species. C. Aneuploidy 1. Aneuploidy is the condition in which an organism gains or loses one or more chromosomes. 2. Monosomy (2n – 1) occurs when an individual has only one of a particular type of chromosome. 3. Trisomy (2n + 1)occurs when an individual has three of a particular type of chromosome. 4. Nondisjunction is the failure of chromosomes to separate at meiosis—both members of the homologous pair go into the same gamete. 5. Monosomy and trisomy occur in plants and animals; in autosomes of animals, it is generally lethal. 6. Trisomy 21 is the most common autosomal trisomy. a. Trisomy 21 (also called Down syndrome) occurs when three copies of chromosome 21 are present. b. Usually two copies of chromosome 21 are contributed by the egg; in 23% of the cases, the sperm had the extra chromosome 21. c. Over 90% of individuals with Down syndrome have three copies of chromosome 21. d. Chances of a woman having a Down syndrome child increase with age, starting at age 40. e. Chorionic villi sampling testing or amniocentesis and karyotyping (see the Science Focus box for a detailed description of this procedure) detects a Down syndrome child; however, risks for young women exceed likelihood of detection. f. A Down syndrome child has many characteristic signs and symptoms, including a tendency for leukemia, cataracts, faster aging, mental retardation, and an increased chance of developing Alzheimer disease later in life. g. The Gart gene, located on the bottom third of chromosome 21, leads to a high level of purines and is associated with the signs and symptoms of Down syndrome; future research may lead to suppression of this gene. D. Changes in Sex Chromosome Number 1. Nondisjunction during oogenesis can result in too few or too many X chromosomes; nondisjunction during spermatogenesis can result in missing or too many Y chromosomes. 2. Turner syndrome females have only one sex chromosome, an X; thus, they are XO, with O signifying the absence of a second sex chromosome. a. Turner females are short, have a broad chest and folds of skin on back of neck. b. Ovaries of Turner females never become functional; therefore, females do not undergo puberty. c. They usually have normal intelligence and can lead fairly normal lives with hormone supplements. 3. Klinefelter syndrome males have one Y chromosome and two or more X chromosomes (e.g., XXY). a. Affected individuals are sterile males; the testes and prostate are underdeveloped. b. Individuals have large hands and feet, long arms and legs, and lack facial hair. c. Presence of the Y chromosome drives male formation but more than two X chromosomes may result in mental retardation. d. A Barr body, usually only seen in the nuclei of a female's cells, is seen in this syndrome due to the two X chromosomes. 4. Poly-X females (or superfemale) have three or more X chromosomes and therefore extra Barr bodies in the nucleus. a. There is no increased femininity; most lack any physical abnormalities. b. XXX individuals are not mentally retarded but may have delayed motor and language development; XXXX females are usually tall and severely mentally retarded. c. Some experience menstrual irregularities but many menstruate regularly and are fertile. 5. Jacobs syndrome (XYY) are males with two Y chromosomes instead of one. a. This results from nondisjunction during spermatogenesis. b. Males are usually taller than average, suffer from persistent acne, and tend to have speech and reading problems. c. Earlier claims that XYY individuals were likely to be aggressive were not correct. 12.4 Changes in Chromosome Structure 1. Environmental factors including radiation, chemicals, and viruses, can cause chromosomes to break; if the broken ends do not rejoin in the same pattern, this causes a change in chromosomal structure. 2. Deletion: a type of mutation in which an end of a chromosome breaks off or when two simultaneous breaks lead to the loss of a segment. 3. Translocation: a chromosomal segment is removed from one chromosome and inserted into another nonhomologous chromosome; in Down syndrome, 5% of cases are due to a translocation between chromosome 21 and 14, a situation that runs in the family of the father or mother. 4. Duplication: the presence of a chromosomal segment more than once on the same chromosome. a. A broken segment from one chromosome can simply attach to its homologue or unequal crossing-over may occur. b. A duplication may also involve an inversion where a segment that has become separated from the chromosome is reinserted at the same place but in reverse; the position and sequence of genes are altered. B. Human Syndromes 1. Deletion Syndromes a. Williams syndrome occurs when chromosome 7 loses an end piece: children look like pixies, have poor academic skills but good verbal and musical skills; lack of elastin causes cardiovascular problems and skin aging. b. Cri du chat syndrome ("cry of the cat") is a deletion in which an individual has a small head, is mentally retarded, has facial abnormalities, and an abnormal glottis and larynx resulting in a cry resembling that of a cat. 2. Translocation Syndromes a. If a translocation results in the normal amount of genetic material, the person will remain healthy; if a person inherits only one of the translocated chromosomes, that person may have only one allele or three alleles rather than the normal two. b. In Alagille syndrome, chromosomes 2 and 20 exchange segments, causing a small deletion on chromosome 20 that may produce some abnormalities. For chapter 16 the best videos to watch the Hershey-Chase video, the videos on DNA and RNA structure in DNA replication. There is a significant amount of detailed history with names of scientists that begin this chapter do your best to remember a few especially Hershey Chase, Chargaff, Franklin and Watson and Crick. 16 - The Genetic Material Early researchers knew that the genetic material must be: 1. able to store information used to control both the development and the metabolic activities of cells; 2. stable so it can be replicated accurately during cell division and be transmitted for generations; and, 3. able to undergo mutations providing the genetic variability required for evolution. Previous Knowledge About DNA 1. Understanding the chemistry of DNA was essential to the discovery that DNA is genetic material. 2. Friedrich Miescher (1869) removed nuclei from pus cells and isolated DNA "nuclein"; it was rich in phosphorus and lacked sulfur. 3. Subsequent analysis of nuclein found that it contained an acidic substance: named it nucleic acid. 4. Two types of nucleic acids were soon discovered: DNA (deoxyribonucleic acid) and RNA (ribonucleic acid). 5. In the early twentieth century, it was shown that nucleic acids contain four types of nucleotides. a. DNA was composed of repeating units, each of which always had just one of each of four different nucleotides (a nitrogenous base, a phosphate, and a pentose). b. In this model, DNA could not vary between species and therefore could not be the genetic material; therefore some other protein component was expected to be the genetic material. A. Transformation of Bacteria 1. Bacteriologist Frederick Griffith (1931) experimented with Streptococcus pneumoniae (a pneumococcus that causes pneumonia in mammals). 2. Mice were injected with two strains of pneumococcus: an encapsulated (S) strain and a nonencapsulated (R) strain. a. The S strain is virulent (the mice died); it has a mucous capsule and forms "shiny" colonies. b. The R strain is not virulent (the mice lived); it has no capsule and forms "dull" colonies. 3. In an effort to determine if the capsule alone was responsible for the virulence of the S strain, he injected mice with heat-killed S strain bacteria; the mice lived. 4. Finally, he injected mice with a mixture of heat-killed S strain and live R strain bacteria. a. The mice died; living S strain pneumococcus were recovered from their bodies. b. Griffith concluded that some substance necessary for synthesis of the capsule--and therefore for virulence--must pass from dead S strain bacteria to living R strain bacteria so the R strain were transformed. c. This change in phenotype of the R strain must be due to a change in the bacterial genotype, suggesting that the transforming substance passed from S strain to R strain. B. DNA: The Transforming Substance 1. Oswald Avery et al. (1944) reported that the transforming substance was DNA. 2. Purified DNA is capable of bringing about the transformation. Evidence: a. DNA from S strain pneumococcus causes R strain bacteria to be transformed. b. Enzymes that degrade proteins cannot prevent transformation, nor can enzymes that digest RNA. c. Digestion of the transforming substance with enzyme that digests DNA prevents transformation. d. The molecular weight of the transforming substance is great enough for some genetic variability. 3. Avery's experimental results demonstrated DNA is genetic material and DNA controls biosynthetic properties of a cell. C. Transformation Experiments Today 1. Transformation experiments today are common in teaching and research labs. 2. Transformation occurs when organisms receive foreign DNA and thereby receive a new trait. 3. Studies with bacteria show some can take up DNA from the medium and thereby gain penicillin resistance. D. Reproduction of Viruses 1. Bacteriophages are viruses that infect bacteria; they consist of a protein coat surrounding a nucleic acid. 2. Bacteriophage T2 is a virus that infects the Escherichia coli (E. coli), a species of bacteria that normally lives within the human gut. 3. Alfred Hershey and Martha Chase (1952) studied bacteriophage T2. a. The purpose of their experiments was to see which of the bacteriophage components— the protein coat or the DNA—entered bacterial cells and directed reproduction of the virus. b. They labeled the protein coat with 35S and the DNA with 32P. c. Viral coats were removed from the bacterial cells and separated by centrifugation. d. Results: radioactive 32P alone is taken up by bacterial host and incorporated in virus reproduction. e. This result reinforced the notion that DNA (and not the protein) is the genetic material. The Structure of DNA A. Nucleotide Data 1. Erwin Chargaff (1940s) analyzed the base content of DNA. 2. It was known DNA contained four different nucleotides: a. two with purine bases, adenine (A) and guanine(G); a purine is a type of nitrogencontaining base having a double-ring structure. b. two with pyrimidine bases, thymine (T) and cytosine (C); a pyrimidine is a type of nitrogen-containing base having a single-ring structure. 3. Results: DNA does have the variability necessary for the genetic material, and, 4. For a species, DNA has the constancy required of genetic material. 5. This constancy is given in Chargaff's rules: a. The amount of A, T, G, and C in DNA varies from species to species. b. In each species, the amount of A = T and the amount of G = C (A +G = T +C). 6. The tetranucleotide hypothesis (proposing DNA was repeating units of one of four bases) was disproved: each species has its own constant base composition. B. Variation in Base Sequence 1. The variability is staggering; a human chromosome contains about 140 million base pairs. 2. Since any of the four possible nucleotides can be present at each nucleotide position, the total number of possible nucleotide sequences is 4140 x 106 = 4140,000,000. C. Diffraction Data 1. Rosalind Franklin produced X-ray diffraction photographs. 2. Franklin's work provided evidence that DNA had the following features: a. DNA is a helix. b. Some portion of the helix is repeated. D. The Watson and Crick Model 1. American James Watson joined with Francis H. C. Crick in England to work on the structure of DNA. 2. Watson and Crick received the Nobel Prize in 1962 for their model of DNA. 3. Using information generated by Chargaff and Franklin, Watson and Crick constructed a model of DNA as a double helix with sugar-phosphate groups on the outside, and paired bases on the inside. 4. Their model was consistent with both Chargaff's rules and Franklin's X-ray diffraction studies. 5. Complementary base pairing is the paired relationship between purines and pyrimidines in DNA: A is hydrogen-bonded to T and G is hydrogen-bonded to C. Replication of DNA DNA replication is the process of copying a DNA molecule. Replication is semiconservative, with each strand of the original double helix (parental molecule) serving as a template (mold or model) for a new strand in a daughter molecule. This process consists of: 1. Unwinding: old strands of the parent DNA molecule are unwound as weak hydrogen bonds between the paired bases are "unzipped" and broken by the enzyme helicase. 2. Complementary base pairing: free nucleotides present in the nucleus bind with complementary bases on unzipped portions of the two strands of DNA; this process is catalyzed by DNA polymerase. 3. Joining: complementary nucleotides bond to each other to form new strands; each daughter DNA molecule contains an old strand and a new strand; this process is also catalyzed by DNA polymerase. 4. DNA replication must occur before a cell can divide; in cancer, drugs with molecules similar to the four nucleotides are used to stop replication. A. Replication is Semiconservative 1. DNA replication is semiconservative: each daughter double helix has one parental strand and one new strand. 2. Matthew Meselson and Franklin Stahl (1958) confirmed the process of DNA replication. a. They grew bacteria in a medium with heavy nitrogen (15N), then switched to light nitrogen (14N). b. The density of DNA following replication is intermediate as measured by centrifugation of molecules. c. After one division, only "hybrid" DNA molecules were in the cells. d. After two divisions, half the DNA molecules were "light" and half were "hybrid." 3. These were exactly the results to be expected if DNA replication is semiconservative. B. Prokaryotic Versus Eukaryotic Replication 1. Prokaryotic Replication a. Bacteria have a single loop of DNA that must replicate before the cell divides. b. Replication in prokaryotes may be bidirectional from one point of origin or in only one direction. c. Replication only proceeds in one direction, from 5' to 3'. d. Bacterial cells are able to replicate their DNA at a rate of about 106 base pairs per minute. e. Bacterial cells can complete DNA replication in 40 minutes; eukaryotes take hours. 2. Eukaryotic Replication a. Replication in eukaryotes starts at many points of origin and spreads with many replication bubbles—places where the DNA strands are separating and replication is occurring. b. Replication forks are the V-shape ends of the replication bubbles; the sites of DNA replication. c. Eukaryotes replicate their DNA at a slower rate – 500 to 5,000 base pairs per minute. d. Eukaryotes take hours to complete DNA replication. C. Replication Errors 1. A genetic mutation is a permanent change in the sequence of bases. 2. Base changes during replication are one way mutations occur. 3. A mismatched nucleotide may occur once per 100,000 base pairs, causing a pause in replication. 4. Proofreading is the removal of a mismatched nucleotide; DNA repair enzymes perform this proofreading function and reduce the error rate to one per billion base pairs. 5. Incorrect base pairs that survive the proofreading process contribute to gene mutations. Make sure you understand the activity on the overview protein synthesis in the MP3 tutor on DNA to RNA to protein is a useful activity to watch. Be sure to understand how RNA is processed in an understanding of mutations, that is gene mutations, is important. 17 - The Function of Genes - from gene and protein 1. Sir Archibald Garrod (early 1900s) introduced the phrase inborn error of metabolism. a. Garrod proposed that inherited defects could be caused by the lack of a particular enzyme. b. Knowing that enzymes are proteins, Garrod suggested a link between genes and proteins. B. Genes Specify Enzymes 1. George Beadle and Edward Tatum (1940) X-rayed spores of the red bread mold, Neurospora crassa. 2. They observed that some resulting cultures lacked a particular enzyme for growth on minimal medium. 3. They found that a single gene was mutated, which resulted in the lack of a single enzyme. 4. They proposed the one gene–one enzyme hypothesis: one gene specifies the synthesis of one enzyme. C. Genes Specify a Polypeptide 1. Linus Pauling and Harvey Itano (1949) compared hemoglobin in red blood cells of persons with sickle-cell disease and normal individuals. 2. They discovered that the chemical properties of a protein chain of sickle-cell hemoglobin differed from that of normal hemoglobin. 3. Vernon Ingram subsequently showed that the biochemical difference in the protein chain of sickle-cell hemoglobin is the substitution of a nonpolar amino acid valine for the negatively charged amino acid glutamate. 4. Pauling and Itano formulated the one gene–one polypeptide hypothesis: each gene specifies one polypeptide of a protein, a molecule that may contain one or more different polypeptides. D. From DNA to RNA to Protein 1. A gene is a sequence of DNA nucleotide bases that codes for a sequence of nucleotides in an RNA molecule. 2. DNA is restricted to nucleus; protein synthesis occurs at ribosomes in the cytoplasm. 3. Ribonucleic acid (RNA) is found in both regions of the cell. E. Types of RNA 1. Like DNA, RNA is a polymer of nucleotides. 2. Unlike DNA, RNA is single-stranded, contains the sugar ribose, and the base uracil instead of thymine (in addition to cytosine, guanine, and adenine). 3. There are three major classes of RNA. a. Messenger RNA (mRNA) takes a message from DNA in the nucleus to ribosomes in the cytoplasm. b. Ribosomal RNA (rRNA) and proteins make up ribosomes where proteins are synthesized. c. Transfer RNA (tRNA) transfers a particular amino acid to a ribosome. F. Gene Expression 1. DNA undergoes transcription to mRNA, which is translated to a protein. 2. DNA is a template for RNA formation during transcription. 3. Transcription is the first step in gene expression; it is the process whereby a DNA strand serves as a template for the formation of mRNA. 4. During translation, an mRNA transcript directs the sequence of amino acids in a polypeptide. The Genetic Code 1. The central dogma of molecular biology states that the sequence of nucleotides in DNA specifies the order of amino acids in a polypeptide. 2. The genetic code is a triplet code, comprised of three-base code words (e.g., AUG). 3. A codon consists of 3 nucleotide bases of DNA. 4. Four nucleotides based on 3-unit codons allows up to 64 different amino acids to the specified. B. Finding the Genetic Code 1. Marshall Nirenberg and J. Heinrich Matthei (1961) found that an enzyme that could be used to construct synthetic RNA in a cell-free system; they showed the codon UUU coded for phenylalanine. 2. By translating just three nucleotides at a time, they assigned an amino acid to each of the RNA codons, and discovered important properties of the genetic code. 3. The code is degenerate: there are 64 triplets to code for 20 naturally occurring amino acids; this protects against potentially harmful mutations. 4. The genetic code is unambiguous; each triplet codon specifies one and only one amino acid. 5. The code has start and stop signals: there are one start codon and three stop codons. C. The Code Is Universal 1. The few exceptions to universality of the genetic code suggests the code dates back to the very first organisms and that all organisms are related. 2. Once the code was established, changes would be disruptive. First Step: Transcription A. Messenger RNA is Formed 1. A segment of the DNA helix unwinds and unzips. 2. Transcription begins when RNA polymerase attaches to a promoter on DNA. A promoter is a region of DNA which defines the start of the gene, the direction of transcription, and the strand to be transcribed. 3. As RNA polymerase (an enzyme that speeds formation of RNA from a DNA template) moves along the template strand of the DNA, complementary RNA nucleotides are paired with DNA nucleotides of the codingstrand. The strand of DNA not being transcribed is called the noncodingstrand. 4. RNA polymerase adds nucleotides to the 3'-end of the polymer under construction. Thus, RNA synthesis is in the 5'-to-3' direction. 5. The RNA/DNA association is not as stable as the DNA double helix; therefore, only the newest portion of the RNA molecule associated with RNA polymerase is bound to DNA; the rest dangles off to the side. 6. Elongation of mRNA continues until RNA polymerase comes to a stop sequence. 7. The stop sequence causes RNA polymerase to stop transcribing DNA and to release the mRNA transcript. 8. Many RNA polymerase molecules work to produce mRNA from the same DNA region at the same time. 9. Cells produce thousands of copies of the same mRNA molecule and many copies of the same protein in a shorter period of time than if a single copy of RNA were used to direct protein synthesis. B. RNA Molecules Are Processed 1. Newly formed primary mRNA transcript is processed before leaving the nucleus. 2. Primary mRNA transcript is the immediate product of transcription; it contains exons and introns. 3. The ends of the mRNA molecule are altered: a cap is put on the 5' end and a poly-A tail is put on the 3' end. a. The "cap" is a modified guanine (G) where a ribosome attaches to begin translation. b. The "poly-A tail" consists of a 150–200 adenine (A) nucleotide chain that facilitates transport of mRNA out of the nucleus and inhibits enzymatic degradation of mRNA. 4. Portions of the primary mRNA transcript, called introns, are removed. a. An exon is a portion of the DNA code in the primary mRNA transcript eventually expressed in the final polypeptide product. b. An intron is a non-coding segment of DNA removed by spliceosomes before the mRNA leaves the nucleus. 5. Ribozymes are RNAs with an enzymatic function restricted to removing introns from themselves. a. RNA could have served as both genetic material and as the first enzymes in early life forms. 6. Spliceosomes are complexes that contains several kinds of ribonucleoproteins. a. Spliceosomes cut the primary mRNA transcript and then rejoin adjacent exons. C. Function of Introns 1. In humans, introns comprise 95% of the average protein-coding gene. 2. Possibly introns divide a gene into regions that can be joined in different combinations for different products. 3. Introns may function to determine which genes are to be expressed and how they should be spliced. Second Step: Translation 1. Translation takes place in the cytoplasm of eukaryotic cells. 2. Translation is the second step by which gene expression leads to protein synthesis. 3. One language (nucleic acids) is translated into another language (protein). B. The Role of Transfer RNA 1. transfer RNA (tRNA) molecules transfer amino acids to the ribosomes. 2. The tRNA is a single-stranded ribonucleic acid that doubles back on itself to create regions where complementary bases are hydrogen-bonded to one another. 3. The amino acid binds to the 3' end; the opposite end of the molecule contains an anticodon that binds to the mRNA codon in a complementary fashion. 4. There is at least one tRNA molecule for each of the 20 amino acids found in proteins. 5. There are fewer tRNAs than codons because some tRNAs pair with more than one codon; if an anticodon contains a U in the third position, it will pair with either an A or G–this is called the wobble hypothesis. 6. The tRNA synthetases are amino acid-activating enzymes that recognize which amino acid should join which tRNA molecule, and covalently joins them. This requires ATP. 7. An amino acid–tRNA complex forms, which then travels to a ribosome to "transfer" its amino acid during protein synthesis. C. The Role of Ribosomal RNA 1. Ribosomal RNA (rRNA) is produced from a DNA template in the nucleolus of the nucleus. 2. The rRNA is packaged with a variety of proteins into ribosomal subunits, one larger than the other. 3. Subunits move separately through nuclear envelope pores into the cytoplasm where they combine when translation begins. 4. Ribosomes can float free in cytosol or attach to endoplasmic reticulum. 5. Prokaryotic cells contain about 10,000 ribosomes; eukaryotic cells contain many times more. 6. Ribosomes have a binding site for mRNA and binding sites for two tRNA molecules. 7. They facilitate complementary base pairing between tRNA anticodons and mRNA codons; rRNA acts as an enzyme (ribozyme) that joins amino acids together by means of a peptide bond. 8. A ribosome moves down the mRNA molecule, new tRNAs arrive, the amino acids join, and a polypeptide forms. 9. Translation terminates once the polypeptide is formed; the ribosome then dissociates into its two subunits. 10. Polyribosomes are clusters of several ribosomes synthesizing the same protein. 11. To get from a polypeptide to a function protein requires correct bending and twisting; chaperonemolecules assure that the final protein develops the correct shape. 12. Some proteins contain more than one polypeptide; they must be joined to achieve the final threedimensional shape. D. Translation Requires Three Steps 1. During translation, mRNA codons base-pair with tRNA anticodons carrying specific amino acids. 2. Codon order determines the order of tRNA molecules and the sequence of amino acids in polypeptides. 3. Protein synthesis involves initiation, elongation, and termination. 4. Enzymes are required for all three steps; energy (ATP) is needed for the first two steps. 5. Chain Initiation a. A small ribosomal subunit attaches to mRNA in the vicinity of the start codon (AUG). b. First or initiator tRNA pairs with this codon; then the large ribosomal subunit joins to the small subunit. c. Each ribosome contains three binding sites: the P (for peptide) site, the A (for amino acid) site, and the E (for exit) site. d. e. f. g. The initiator tRNA binds to the P site although it carries one amino acid, methionine. The A site is for the next tRNA carrying the next amino acid. The E site is to discharge tRNAs from the ribosome. Initiation factor proteins are required to bring together the necessary translation components: the small ribosomal subunit, mRNA, initiator tRNA, and the large ribosomal subunit. 6. Chain Elongation a. The tRNA with attached polypeptide is at the P site; a tRNA-amino acid complex arrives at the A site. b. Proteins called elongation factors facilitate complementary base pairing between the tRNA anticodon and the mRNA codon. c. The polypeptide is transferred and attached by a peptide bond to the newly arrived amino acid in the A site. d. This reaction is catalyzed by a ribozyme, which is part of the larger subunit. e. The tRNA molecule in the P site is now empty. f. Translocation occurs with mRNA, along with peptide-bearing tRNA, moving to the P site and the spent tRNA moves from the P site to the E site and exits the ribosome. g. As the ribosome moves forward three nucleotides, there is a new codon now located at the empty A site. h. The complete cycle is rapidly repeated, about 15 times per second in Escherichia coli. 7. Chain Termination a. Termination of polypeptide synthesis occurs at a stop codon that does not code for amino acid. b. The polypeptide is enzymatically cleaved from the last tRNA by a release factor. c. The tRNA and polypeptide leave the ribosome, which dissociates into its two subunits. 8. Definition of a Gene and a Genetic Mutation a. Originally a gene was defined as a locus on the chromosome. b. The one gene-one polypeptide concept connected inborn errors of metabolism with a sequence of DNA bases. c. A gene could also be defined as a sequence of DNA bases coding for a single polypeptide or a single RNA. d. These concepts can allow us to define a mutation as a permanent change in the sequence of DNAbases. e. Current definitions: a protein-coding gene is one that is transcribed into mRNA, while a noncoding gene is one that is transcribed into any other type of RNA. E. Protein Synthesis and the Eukaryotic Cell 1. The first few amino acids of a polypeptide act as a signal peptide that indicates where the polypeptide belongs in the cell or if it is to be secreted by the cell. 2. After the polypeptide enters the lumen of the ER, it is folded and further processed by addition of sugars, phosphates, or lipids. 3. Transport vesicles carry the proteins between organelles and to the plasma membrane. Chapter 18 - regulation of gene expression. Be sure to check out the MP3 tutor control of gene expression, and see activities on lack operon in the methods of eukaryotic gene expression. Remember that the lack operon and TRP operon are very similar so knowing one should allow you to understand the other. Prokaryotic Regulation 1. Bacteria do not require the same enzymes all the time; they produce just those needed at the moment. 2. Francois Jacob and Jacques Monod (1961) proposed the operon model to explain regulation of gene expression in prokaryotes. a. In the operon model, several genes code for an enzyme in the same metabolic pathway and are located in a sequence on a chromosome; expression of structural genes is controlled by the same regulatory genes. b. An operon is a group of structural and regulatory genes that function as a single unit; it includes the following: i. A regulator gene, located outside the operon, codes for a repressor protein molecule that controls whether the operon is active or not. ii. A promotor is the sequence of DNA where RNA polymerase attaches when a gene is to be transcribed. iii. An operator is a short sequence of DNA where an active repressor binds, preventing RNA polymerase from attaching to the promotor--transcription therefore does not occur. iv. Structural genes are one to several genes coding for enzymes of a metabolic pathway that are transcribed as a unit. B. The trp Operon 1. Some operons in E. coli usually exist in the "on" rather than the "off" condition. 2. E. coli produces five enzymes as part of the anabolic pathway to synthesize the amino acid tryptophan. 3. If tryptophan is already present in medium, these enzymes are not needed and the operon is turned off . a. The regulator codes for a repressor that usually is unable to attach to the operator. b. The repressor has a binding site for tryptophan (if tryptophan is present, it binds to the repressor). c. This changes the shape of the repressor that now binds to the operator. 4. The entire unit is called a repressible operon; tryptophan is the corepressor. 5. Repressible operons are involved in anabolic pathways that synthesize substances needed by cells. C. The lac Operon 1. If E. coli is denied glucose and given lactose instead, it makes three enzymes to metabolize the lactose. 2. These three enzymes are encoded by three genes. a. One gene codes for beta-galactosidase that breaks lactose to glucose and galactose. b. A second gene codes for a permease that facilitates entry of lactose into the cell. c. A third gene codes for enzyme transacetylase, which is an accessory in lactose metabolism. 3. The three genes are adjacent on a chromosome and under control of one promoter and one operator. 4. The regulator gene codes for a lac operon repressor protein that binds to the operator and prevents transcription of the three genes. 5. When E. coli is switched to medium containing an allolactose, this lactose binds to the repressor and the repressor undergoes a change in shape that prevents it from binding to the operator. 6. Because the repressor is unable to bind to the operator, the promoter is able to bind to RNA polymerase, which carries out transcription and produces the three enzymes. 7. An inducer is any substance (lactose in the case of the lac operon) that can bind to a particular repressor protein, preventing the repressor from binding to a particular operator; consequently, RNA polymerase can bind to the promoter and transcribe the structural genes. D. Further Control of the lac Operon 1. Since E. coli prefers to break down glucose, how does E. coli know how to turn on when glucose is absent? 2. When glucose is absent, cyclic AMP (cAMP) accumulates; cAMP has only one phosphate group and attaches to ribose at two locations. a. CAP is a catabolite activator protein (CAP) in the cytoplasm. b. When cAMP binds to CAP, the complex attaches to a CAP binding site next to the lac promoter. c. When CAP binds to DNA, DNA bends, exposing the promoter to RNA polymerase. d. Only then does RNA polymerase bind to the promoter; this allows expression of the lac operon structural genes. 3. When glucose is present, there is little cAMP in the cell. a. CAP is inactive and the lactose operon does not function maximally. b. CAP affects other operons when glucose is absent. c. This encourages metabolism of lactose and provides a backup system for when glucose is absent. E. Negative Versus Positive Control 1. Active repressors shut down the activity of an operon—this is negative control.. 2. CAP is an example of positive control; when the molecule is active, it promotes the activity of the operon. 3. Use of both positive and negative controls allows the cell to fine-tune control of its metabolism. 4. If both glucose and lactose are present, the cell preferentially metabolizes glucose. Eukaryotic Regulation 1. Different cells in the human body turn on different genes that code for different protein products. 2. Eukaryotes have four levels of regulatory mechanisms to control gene expression; two in the nucleus and two in the cytoplasm. 3. There are several levels of control that can modify the amount of gene product. a. Chromatin structure: if genes are not accessible to RNA polymerase, they cannot be transcribed. i. Chromatin structure is part of epigenetic inheritance, the transmission of genetic information outside the coding sequences of a gene. b. Transcriptional control in the nucleus determines which structural genes are transcribed and the rate of transcription; it includes transcription factors initiating transcription and transposons (DNA sequences that move between chromosomes and shut down genes). c. Posttranscriptional control occurs in the nucleus after DNA is transcribed and preliminary mRNA forms. i. This may involve differential processing of mRNA before it leaves the nucleus. ii. The speed that mature mRNA leaves nucleus affects the ultimate amount of gene product. d. Translational control occurs in cytoplasm after mRNA leaves the nucleus but before there is a protein product. i. The life expectancy of mRNA molecules can vary, as well as their ability to bind ribosomes. ii. Some mRNAs may need additional changes before they are translated at all. e. Posttranslational control occurs in the cytoplasm after protein synthesis. i. Polypeptide products may undergo additional changes before they are biologically functional. ii. A functional enzyme is subject to feedback control; binding of an end product can change the shape of an enzyme so it no longer carries out its reaction. B. Chromatin Structure 1. Eukaryotic DNA is in the form of chromatin, a stringy material associated with proteins. 2. The DNA is wound around a core of eight protein molecules ("beads on a string"); the proteins are called histones and each "bead" is called a nucleosome. 3. During interphase, some chromatin is highly compact, darkly stained, and genetically inactive heterochromatin. 4. The rest is diffuse, lightly colored euchromatin thought to be genetically active. 5. Barr bodies are an example of heterochromatin. a. Since human males have only one X chromosome, it might be supposed that they produce half the gene product of a female with two X chromosomes. b. However, females have in each nucleated cell a darkly staining Barr body, a condensed, inactive X chromosome. c. Which X chromosome is condensed in each cell is determined by chance. d. Thus, the body of heterozygous females is "mosaic"; half her cells express alleles on one X chromosome and half of her cells express the alleles on the other X chromosome. e. Female gonads do not show Barr bodies; both X chromosomes are needed in development. f. Only one active X chromosome in the female zygote means that lower X-coded gene products are normal. g. Other examples of this mosaic effect include: ocular albinism, Duchenne muscular dystrophy, and female calico cat coat color. 6. Euchromatin activity is related to the extent nucleosomes are coiled and condensed. a. A nucleosome is a bead-like unit made of a segment of DNA wound around a complex of histone proteins. b. When DNA is transcribed, activators called remodeling proteins are able to push aside the histone proteins so transcription can begin. 7. Epigenetic inheritance is the term used to describe inheritance patterns that do not depend on the genes themselves. a. Histones proteins have different chemical modifications in heterochromatin and euchromatin. b. Methylation of DNA accounts for a phenomenon called genomic imprinting: gene expression is dependent on whether the chromosome carrying the gene is inherited from the mother or the father. c. Epigenetic inheritance explains unusual inheritance patterns, and may have implications in growth, aging and cancer. C. Transcriptional Control 1. Transcriptional control is the most critical level of genetic control. a. Transcription is controlled by DNA-binding proteins called transcription factors. b. Each cell contains many different types of transcription factors. c. A group of transcription factors binds to a promoter adjacent to a gene; the complex attracts and binds RNA polymerase, but transcription may still not begin. d. Transcription activators are often involved in controlling transcription in eukaryotes. i. Different combinations may regulate different genes. ii. Transcription activators bind to DNA regions called enhancers. iii. Enhancers can be quite a distance from the promoter, but a hairpin loop in the DNA brings the activator attached to an enhancer into contact with the promotor. iv. Mediator proteins act as a bridge between transcription factors and transcription factors at the promotor. e. Transcription factors are always present in the cell and most likely they have to be activated in some way (e.g., regulatory pathways involving kinases or phosphatases) before they bind to DNA. 2. Transposons are specific DNA sequences that move with and between chromosomes. a. Their movement may increase or decrease the expression of neighboring genes. b. They are among the 40% of the human genome consisting of the same short sequence of DNA continuously repeated. c. They are noncoding sequences that play regulatory functions, and could thus be cansidered part of epigenetic inheritance. D. Posttranscriptional Control 1. Posttranscriptional control includes mRNA processing and the speed at which mRNA leaves the nucleus. 2. Messenger RNA molecules are processed before they leave the nucleus and enter the cytoplasm. 3. Differential excision of introns and splicing of mRNA can vary the type of mRNA that leaves nucleus. a. The hypothalamus and thyroid glands produce calcitonin but the mRNA that leaves the nucleus is not the same in both types of cells. b. Radioactive labeling shows they vary because of a difference in mRNA splicing. c. Evidence of different patterns of mRNA splicing is found in cells that produce neurotransmitters, muscle regulatory proteins, and antibodies. 4. Speed of transport of mRNA from nucleus into cytoplasm affects the amount of gene product realized per unit time following transcription; there is a difference in the length of time it takes various mRNA molecules to pass through nuclear pores. E. Translational Control 1. Translational control begins when the processed mRNA molecule reaches the cytoplasm and before there is a protein product. a. The longer an active mRNA molecule remains in the cytoplasm, the more product is produced. b. Mature red blood cells eject their nucleus but synthesize hemoglobin for several months; the mRNAs must persist during this time. c. Ribonucleases are enzymes associated with ribosomes that degrade mRNA. d. Mature mRNA has non-coding segments at 3' cap and 5' poly-A tail ends; differences in these segments influence how long the mRNA avoids being degraded. e. MicroRNAs are small, processed pieces of intron; after microRNAs are degraded, they combine with protein and the complex binds to mRNAs, destroying them. F. Posttranslational Control 1. Posttranslational control begins once a protein has been synthesized and has become active. a. Some proteins are not active after synthesis; the polypeptide product has to undergo additional changes before it is biologically functional. b. Bovine proinsulin, for example, is inactive when first produced; a single long polypeptide folds into a three-dimensional structure, a sequence of 30 amino acids is removed from the middle, and the two polypeptide chains are bonded together by disulfide bonds resulting in an active protein. c. Many proteins are short-lived in cells and degraded or destroyed so they are no longer active. d. Giant protein complexes called proteasomes carry out this task. e. One example is the cyclins that control the cell cycle; they are only temporarily present.. Genetic Mutations A genetic mutation is a permanent change in the sequence of bases in DNA; mutations range from having no effect to total inactivity. A. Effect of Mutations on Protein Activity 1. Point mutations change a single nucleotide and therefore change a single specific codon. a. The effect of the point mutation depends on the specific base change in the codon. b. Changes to codons that code for the same amino acid have no effect; e.g., UAU to UAC both code for tyrosine. c. A change from UAC to UAG (a stop codon) results in a shorter protein, and a change from UAC to CAC incorporates histidine instead of tyrosine. d. Sickle cell disease results from a single base change in DNA where the beta-chain of hemoglobin contains valine instead of glutamate at one location and the resulting distorted hemoglobin causes red blood cells to clog vessels and die off sooner. 2. Frameshift Mutations a. The reading frame depends on the sequence of codons from the starting point: THE CAT ATE THE RAT. b. If, for example, C is deleted, the reading frame is shifted: THE ATA TET HER AT. c. Frameshift mutations occur when one or more nucleotides are inserted or deleted from DNA. d. The result of a frameshift mutation is a new sequence of codons and nonfunctional proteins. B. Nonfunctional Proteins 1. A single nonfunctioning protein can cause dramatic effects. 2. The human transposon Alu is responsible for hemophilia when it places a premature stop codon in the gene for clotting factor IX. 3. PKU results when a person cannot convert phenylalanine to tyrosine; phenylalanine builds up in the system, leading to mental retardation. 4. A faulty code for an enzyme in the same pathway results in an albino individual. 5. Cystic fibrosis is due to inheriting a faulty code for a chloride transport protein in the plasma membrane. 6. Androgen insensitivity is due to a faulty receptor for male sex hormones; body cells cannot respond to testosterone and the individual develops as a female (even though all of the body cells are XY). C. Carcinogenesis 1. The development of cancer involves a series of various types of mutations. 2. Tumor-suppressor genes normally act as brakes on cell division when it begins to occur abnormally. 3. When proto-oncogenes mutate, they become oncogenes. 4. Tumor-suppressor genes and proto-oncogenes often code for transcription factors or proteins that control transcription factors. 5. P53, a major tumor-suppressor gene, is more frequently mutated in human cancers than any other known gene. a. The p53 protein acts as a transcription factor to turn on the expression of genes whose products are cell cycle inhibitors. b. The p53 can also stimulate apoptosis (programmed cell death). 6. Other proto-oncogenes code for Ras proteins, which are needed for normal cell growth and DNA synthesis. 7. Inheritance of Cancer a. Genes called BRCA1and BRCA2 are tumor-suppressor genes that behave as outosomal recessive alleles. b. A mutated RB allele causes cancer of the eye; it takes another mutated allele to increase the chance of cancer. c. The inherited RET gene predisposes a person to thyroid cancer. D. Cause of Mutations 1. Some mutations are spontaneous, others are due to environmental mutagens. 2. Mutations due to replication errors are very rare. 3. DNA polymerase constantly proofreads new DNA against the old, and repairs any irregularities, thereby reducing mistakes to one out of every one billion nucleotide pairs replicated. 4. Environmental mutagens are environmental substances that increase the chances of mutation. a. Common mutagens are radiation and organic chemicals. b. Carcinogens are mutagens that increase the chances of cancer. i. The Ames test is commonly-used to determine if a chemical is carcinogenic. ii. A histidine-requiring strain of bacteria is exposed to a chemical. iii. If the chemical is mutagenic, the bacteria regain the ability to grow without histidine. c. Tobacco smoke contains a number of known carcinogenic chemicals. d. X rays and gamma rays are ionizing radiation that creates free radicals, ionized atoms with unpaired electrons. e. Ultraviolet (UV) radiation is easily absorbed by pyrimidines in DNA. i. Where two thymine molecules are near each other, UV may bond them together as thymine dimers. ii. Usually dimers are removed from damaged DNA by special enzymes called repair enzymes. 20 biotechnology - this unit is complex so you need to have a basic understanding of how DNA replicates and how proteins are constructed. It is vital to understand how DNA replicates itself because many of the molecules in DNA replication are used in biotechnology. Be sure to review the application of DNA technology video along with the videos on restriction enzymes, gel electrophoresis and the human genome project. Cloning is the production of identical copies of DNA through some asexual means. a. An underground stem or root sends up new shoots that are clones of the parent plant. b. Members of a bacterial colony on a petri dish are clones because they all came from division of the same cell. c. Human identical twins are clones; the original single embryo separate to become two individuals. 2. Gene cloning is production of many identical copies of the same gene. a. If the inserted gene is replicated and expressed, we can recover the cloned gene or protein product. b. Cloned genes have many research purposes: determining the base sequence between normal and mutated genes, altering the phenotype, obtaining the protein coded by a specific gene, etc. c. Humans can be treated with gene therapy: alteration of the phenotype in a beneficial way. B. Recombinant DNA Technology 1. Recombinant DNA (rDNA) contains DNA from two or more different sources. 2. To make rDNA, technician selects a vector. 3. A vector is a plasmid or a virus used to transfer foreign genetic material into a cell. 4. A plasmid is a small accessory ring of DNA in the cytoplasm of some bacteria. 5. Plasmids were discovered in research on reproduction of intestinal bacteria Escherichia coli. 6. Introduction of foreign DNA into vector DNA to produce rDNA requires two enzymes. a. Restriction enzyme is a bacterial enzyme that stops viral reproduction by cleaving viral DNA. i. The restriction enzyme is used to cut DNA at specific points during production of rDNA. ii. It is called a restriction enzyme because it restricts growth of viruses but it acts as a molecular scissors to cleave any piece of DNA at a specific site. iii. Restriction enzymes cleave vector (plasmid) and foreign (human) DNA. iv. Cleaving DNA makes DNA fragments ending in short single-stranded segments with "sticky ends." v. The "sticky ends" allow insertion of foreign DNA into vector DNA. b. DNA ligase seals the foreign gene into the vector DNA i. ii. Treated cells take up plasmids, and then bacteria and plasmids reproduce. Eventually, there are many copies of the plasmid and many copies of the foreign gene. iii. When DNA splicing is complete, an rDNA (recombinant DNA) molecule is formed. 7. If the human gene is to express itself in a bacterium, the gene must be accompanied by the regulatory regions unique to bacteria and meet other requirements. a. The gene cannot contain introns because bacteria do not have introns. b. An enzyme called reverse transcriptase can be used to make a DNA copy of mRNA. c. This DNA molecule is called complementary DNA (cDNA) and does not contain introns. d. A laboratory DNA synthesizer can produce small pieces of DNA without introns. C. The Polymerase Chain Reaction (PCR) 1. PCR can create millions of copies of a single gene or a specific piece of DNA in a test tube. 2. PCR is very specific—the targeted DNA sequence can be less than one part in a million of the total dxcDNA sample; therefore a single gene can be amplified using PCR. 3. PCR uses the enzyme DNA polymerase to carry out multiple replications (a chain reaction) of target DNA. 4. PCR automation is possible because heat-resistant DNA polymerase from Thermus aquaticus, which grows in hot springs, is an enzyme that withstands the temperature necessary to separate double-stranded DNA. 5. Analyzing DNA Segments a. Mitochondria DNA sequences in modern living populations can decipher the evolutionary history of human populations. b. DNA fingerprinting is the technique of using DNA fragment lengths, resulting from restriction enzyme cleavage and amplified by PCR, to identify particular individuals. c. DNA is treated with restriction enzymes to cut it into different sized fragments. d. During gel electrophoresis, fragments separate according to length, resulting in a pattern of bands. e. DNA fingerprinting can identify deceased individuals from skeletal remains, perpetrators of crimes from blood or semen samples, and genetic makeup of long-dead individuals or extinct organisms. 6. PCR amplification and DNA analysis is used to: a. detect viral infections, genetic disorders, and cancer; b. determine the nucleotide sequence of human genes, and, because it is inherited, c. associate samples with DNA of parents. Biotechnology Products 1. Genetically engineered organisms can produce biotechnology products. 2. Organisms that have had a foreign gene inserted into them are transgenic. B. Transgenic Bacteria 1. Bacteria are grown in large vats called bioreactors. a. Foreign genes are inserted and the product is harvested. b. Products on the market include insulin, hepatitis B vaccine, t-PA, and human growth hormone. 2. Transgenic bacteria have been produced to protect and improve the health of plants. a. Frost-minus bacteria protect the vegetative parts of plants from frost damage. b. Root-colonizing bacteria receive genes from bacteria for insect toxin, protecting the roots. c. Bacteria that colonize corn roots can be endowed with genes for insect toxin. 3. Transgenic bacteria can degrade substances. a. Bacteria selected for ability to degrade oil can be improved by genetic engineering. b. Bacteria can be bio-filters to prevent airborne chemical pollutants from being vented into the air. c. Bacteria can also remove sulfur from coal before it is burned and help clean up toxic dumps. d. Bacteria can also be given"suicide genes" that caused them to die after they have done their job. 4. Transgenic bacteria can produce chemical products. a. Genes coding for enzymes can be manipulated to catalyze synthesis of valuable chemicals. b. Phenylalanine used in artificial sweetener can be grown by engineered bacteria. 5. Transgenic bacteria process minerals. a. Many major mining companies already use bacteria to obtain various metals. b. Genetically engineered "bio-leaching" bacteria extract copper, uranium, and gold from low-grade ore. C. Transgenic Plants 1. Plant cells that have had the cell wall removed are called protoplasts. 2. Electric current makes tiny holes in the plasma membrane through which genetic material enters. 3. The protoplasts then develop into mature plants. 4. Foreign genes now give cotton, corn, and potato strains the ability to produce an insect toxin and soybeans are now resistant to a common herbicide. 5. Plants are being engineered to produce human proteins including hormones, clotting factors, and antibodies in their seeds; antibodies made by corn, deliver radioisotopes to tumor cells and a soybean engineered antibody can treat genital herpes. D. Transgenic Animals 1. Animal use requires methods to insert genes into eggs of animals. a. It is possible to microinject foreign genes into eggs by hand. b. Vortex mixing places eggs in an agitator with DNA and silicon-carbide needles that make tiny holes through which the DNA can enter. c. Using this technique, many types of animal eggs have been injected with bovine growth hormone (bGH) to produce larger fishes, cows, pigs, rabbits, and sheep. 2. Gene pharming is the use of transgenic farm animals to produce pharmaceuticals; the product is obtainable from the milk of females. a. Genes for therapeutic proteins are inserted into animal's DNA; animal's milk produces proteins. b. Drugs obtained through gene pharming are planned for the treatment of cystic fibrosis, cancer, blood diseases, and other disorders. E. Cloning Transgenic Animals 1. For many years, it was believed that adult vertebrate animals could not be cloned; the cloning of Dolly in 1997 demonstrated this can be done. 2. Cloning of an adult vertebrate would require that all genes of an adult cell be turned on again. 3. Cloning of mammals involves injecting a 2n nucleus adult cell into an enucleated egg. 4. The cloned eggs begin development in vitro and are then returned to host mothers until the clones are born. Genomics Genetics in the 21st century concerns genomics: the study of genomes of humans and other organisms. A. Sequencing the Bases 1. The Human Genome Project has produced a working draft of all the base pairs in all our chromosomes. B. C. D. E. F. 2. The task took 13 years to learn the sequence of the three billion base pairs along the length of our chromosomes. Genome Comparisons 1. There is little difference between the sequence of our bases and other organisms whose DNA sequences are known. 2. We share a large number of genes with simpler organisms (e.g., bacteria, yeast, mice); perhaps our uniqueness is due to regulation of these genes. 3. Researchers found that certain genes on chromosome 22 differed in humans and chimpanzees: those for speech development, hearing, and smell. 4. Many genes found were responsible for human diseases. The HapMap Project 1. This project will catalog sequence differences, called haplotypes, in humans. 2. The goal of the project is to link haplotypes to the risk for specific illnesses. The Genetic Profile 1. An important aspect of genomics is to determine how genes work together to control the phenotype. 2. DNA chips (or DNA microarrays) will soon be available that will rapidly identify a person's complete genotype; this is called the genetic profile. 3. DNA profiles can determine if a person has an increased risk for a particular disease; appropriate intervention can then be administered. 4. The genetic profile can be used to determine if a particular drug therapy is appropriate in a specific clinical condition. Proteomics 1. Proteomics is the study of the structure, function, and interaction of cellular proteins. 2. The information obtained from proteomic studies can be used in designing better drugs, and to correlate drug treatment to the particular genome of the individual. Bioinformatics 1. Bioinformatics is the application of computer technologics to the study of the genome. 2. Information obtained from computer analysis of the genome can show relationships between genetic profiles and genetic disorders. Gene Therapy 1. Gene therapy involves procedures to give patients healthy genes to make up for a faulty gene. 2. Gene therapy also includes the use of genes to treat genetic disorders and various human illnesses. 3. There are ex vivo (outside body) and in vivo (inside body) methods of gene therapy. B. Ex Vivo Gene Therapy 1. Children with severe combined immunodeficiency (SCID) underwent ex vivo gene therapy. a. Lacking the enzyme ADA involved in maturation of T and B cells, they faced lifethreatening infections. b. Bone marrow stem cells are removed, infected with a retrovirus that carries a normal gene for the enzyme ADA, and returned. c. Use of bone marrow stem cells allows them to divide and produce more cells with the same genes. d. Patients who undergo this procedure show significant improvement. 2. Gene therapy trials include treatment of familial hypercholesterolemia where liver cells lack a receptor for removing cholesterol from blood. a. High levels of blood cholesterol make the patient subject to fatal heart attacks when young. b. A small portion of the liver is surgically removed and infected with retrovirus with normal gene for receptor. c. This has lowered cholesterol levels following the procedure. C. In Vivo Gene Therapy 1. Cystic fibrosis patients lack a gene for trans-membrane chloride ion carriers; patients die from respiratory tract infections. a. Liposomes, microscopic vesicles that form when lipoproteins are in solution, are coated with healthy cystic fibrosis genes and sprayed into a patient's nostrils. b. Various methods of delivery are being tested for effectiveness. 2. A gene for vascular endothelial growth factor (VEGF) can be injected alone or within a virus into the heart to stimulate branching of coronary blood vessels. 3. Another strategy is to make cancer cells more vulnerable, and normal cells more resistant, to chemotherapy. 4. Injecting a retrovirus containing a normal p53 gene–that promotes apoptosis–into tumors may stop the growth of tumors. It is crucial in the evolution unit that you have examples of how evolution has changed various organisms. It would be nice to use the horse, in vertebrate evolution or vertebrate evolution but you need an example of some kind to successfully answer questions in this section. There is a significant amount of history in the beginning of this unit but you need to concentrate on Darwin's theory and try to remember some of the names of the other scientists such as Linnaeus, Lamarck, Lyle and Hutton. 22 - History of Evolutionary Thought - Darwin and the idea of descent with modification. 1. In 1831, Charles Darwin, a 22-year-old naturalist, accepted a position aboard the ship HMS Beagle that began a voyage around the world; it provided Darwin with many observations. 2. The pre-Darwinian world-view was different from the post-Darwinian. a. Pre-Darwinian world-view was determined by intractable theological beliefs. i. The earth is young. ii. Each species was specially created and did not change over time. iii. Variations are imperfections varying from a perfectly-adapted creation. iv. Observations are to substantiate the prevailing worldview. b. Darwin, however, lived during a time of great change in scientific and social realms. c. Darwin's ideas were part of a larger change in thought already underway among biologists; this concept would eventually be known as evolution. B. Mid-Eighteenth-Century Contributions 1. Carolus Linnaeus and Taxonomy a. Taxonomy is the science of classifying organisms; taxonomy had been a main concern of biology. b. Carolus Linnaeus (1707–1778) was a Swedish taxonomist. i. Linnaeus developed a binomial system of nomenclature (two-part names for each species [e.g., Homo sapiens]). ii. He developed a system of classification for all known plants. iii. Like other taxonomists of his time, Linnaeus believed in the ideas of 1. special creation—each species had an "ideal" structure and function; and 2. fixity of species—each species had a place in the scala naturae, a sequential ladder of life. c. Linnaeus thought that classification should describe the fixed features of species and reveal God's divine plan. d. His ideas reflected the ideas of Plato and Aristotle: the ideal form can be deduced, and organisms can be arranged in order of increasing complexity. e. His later work with hybridization suggested species might change with time. 2. Georges Louis Leclerc a. Georges Louis Leclerc, known by his title, Count Buffon (1707–1788), was a French naturalist. b. He wrote a 44-volume natural history of all known plants and animals. c. He also provided evidence of descent with modification. d. His writings speculated on influences of the environment, migration, geographical isolation, and the struggle for existence. e. Buffon vacillated on whether he believed in evolutionary descent and he professed to believe in special creation and the fixity of species. 3. Erasmus Darwin a. Erasmus Darwin (1731–1802) was Charles Darwin's grandfather. b. He was a physician and a naturalist whose writings on both botany and zoology contained many comments that suggested the possibility of common descent. c. He based his conclusions on i. changes undergone by animals during development, ii. artificial selection by humans, and iii. the presence of vestigial organs (organs that are believed to have been functional in an ancestor but are reduced and nonfunctional in a descendant). d. Erasmus Darwin offered no mechanism by which evolutionary descent might occur. C. Late Eighteenth-/Early-Nineteenth Century Contributions 1. Cuvier and Catastrophism a. George Cuvier (1769–1832), a French vertebrate zoologist, was the first to use comparative anatomy to develop a system of classifying animals. b. He founded the science of paleontology—the study of fossils—and suggested that a single fossil bone was all he needed to deduce the entire anatomy of an animal. c. To explain the fossil record, Cuvier proposed that a whole series of catastrophes (extinctions) and re-populations from other regions had occurred. d. Cuvier was also a staunch advocate of special creation and fixity of species; this presented him with a problem when geological evidence of a particular region showed a succession of life forms in the earth's strata. e. Catastrophism is the term applied to Cuvier's explanation of fossil history: the belief that catastrophic extinctions occurred, after which repopulation of surviving species occurred, giving an appearance of change through time. 2. Lamarck's Acquired Characteristics a. Lamarck (1744–1829) was the first to state that descent with modification occurs and that organisms become adapted to their environments. b. Lamarck, an invertebrate zoologist, held ideas at odds with Cuvier's. c. Lamarck mistakenly saw "a desire for perfection" as inherent in all living things. d. Inheritance of acquired characteristics was Lamarck's belief that organisms become adapted to their environment during their lifetime and pass these adaptations to their offspring. e. Experiments fail to uphold Lamarck's inheritance of acquired characteristics; the molecular mechanism of inheritance shows phenotypic changes do not result in genetic changes that can be passed on to the next generation. 17.2 Darwin's Theory of Evolution A. Darwin's Background 1. His nature was too sensitive to pursue medicine; he attended divinity school at Cambridge. 2. He attended biology and geology lectures and was tutored by the Reverend John Henslow. 3. Henslow arranged his five-year trip on the HMS Beagle; Darwin was an observant student of nature. B. Geology and Fossils 1. His study of geology and fossils caused him to concur with Lyell that the observed massive geological changes were caused by slow, continuous processes. a. Darwin took Lyell's book on the voyage of the HMS Beagle. b. In his book Principles of Geology, Charles Lyell presented arguments to support a theory of geological change proposed by James Hutton. c. In contrast to catastrophists, Hutton proposed that the earth was subject to slow but continuous geological processes (e.g., erosion and uplifting) that occur at a uniform rate, a theory called uniformitarianism. d. The Argentina coast had raised beaches; he witnessed earthquakes raising the earth several feet. e. Marine shells occurred far inland and at great heights in the Andes. f. Fossils of huge sloths and armadillo-like animals suggested modern forms were descended from extinct forms with change over time; therefore species were not fixed. C. Biogeography 1. Biogeography is the study of the geographic distribution of life forms on earth. 2. Patagonian hares replaced rabbits in the South American grasslands. 3. The greater rhea found in the north was replaced by the lesser rhea in the south. 4. Comparison of the animals of South America and the Galápagos Islands caused Darwin to conclude that adaptation to the environment can cause diversification, including origin of new species. 5. The Galápagos Islands a. These volcanic islands off the South American coast had fewer types of organisms. b. Island species varied from the mainland species, and from island-to-island. c. Each island had a variation of tortoise; long and short necked tortoises correlated with different vegetation. d. Darwin's Finches 1. Finches on the Galápagos Islands resembled a mainland finch but there were more types. 2. Galápagos finch species varied by nesting site, beak size, and eating habits. 3. One unusual finch used a twig or thorn to pry out insects, a job normally done by (missing) woodpeckers (Darwin never witnessed this finch behavior). 4. The variation in finches posed questions to Darwin: did they descend from one mainland ancestor or did islands allow isolated populations to evolve independently, and could present-day species have resulted from changes occurring in each isolated population? D. Natural Selection and Adaptation 1. Darwin decided that adaptations develop over time; he sought a mechanism by which adaptations might arise. 2. Natural selection was proposed by both Alfred Russel Wallace and Darwin as a driving mechanism of evolution caused by environmental selection of organisms most fit to reproduce, resulting in adaptation. 3. Because the environment is always changing, there is no perfectly-adapted organism. 4. There are three preconditions for natural selection. a. The members of a population have random but heritable variations. b. In a population, many more individuals are produced each generation than the environment can support. c. Some individuals have adaptive characteristics that enable them to survive and reproduce better. 5. There are two consequences of natural selection. a. An increasing proportion of individuals in succeeding generations will have the adaptive characteristics. b. The result of natural selection is a population adapted to its local environment. E. F. G. H. I. 6. Natural selection can only utilize variations that are randomly provided; therefore there is no directedness or anticipation of future needs. 7. Extinction occurs when previous adaptations are no longer suitable to a changed environment. Organisms Have Variations 1. In contrast to the previous worldview where imperfections were to be ignored, variations were essential in natural selection. 2. Darwin suspected, but did not have today's evidence, that the occurrence of variation is completely random. 3. New variations are as likely to be harmful as helpful. 4. Variations that make adaptation possible are those that are passed on from generation to generation. 5. Darwin could not state the cause of variations because genetics was not yet established. Organisms Struggle to Exist 1. Darwin and Wallace both read an essay by Thomas Malthus, a socioeconomist. 2. Malthus proposed that human populations outgrow food supply and death and famine were inevitable. 3. Darwin applied this to all organisms; resources were not sufficient for all members to survive. 4. Therefore, there is a constant struggle for existence; only certain members survive and reproduce. Organisms Differ in Fitness 1. Organisms whose traits enable them to reproduce to a greater degree have a greater fitness. a. Fitness is a measure of an organism's reproductive success. b. Black western diamondback rattlesnakes are more likely to survive on lava flows; lightercolored rattlesnakes are more likely to survive on desert soil. 2. Darwin noted that humans carry out artificial selection. a. Early humans likely selected wolf variants; consequently, desirable traits increase in frequency in subsequent generations and produced the varieties of domestic dogs. b. Many crop plant varieties can be traced to a single ancestor. c. In nature, interactions with the environment determine which members reproduce more. d. Evolution by artificial or natural selection occurs when more fit organisms reproduce and leave more offspring than the less fit. Organisms Become Adapted 1. An adaptation is a trait that helps an organism be more suited to its environment. 2. Unrelated organisms living in the same environment often display similar characteristics. 3. Because of differential reproduction, adaptive traits increase in each succeeding generation. On the Origin of Species by Darwin 1. After the HMS Beagle returned to England in 1836, Darwin waited over 20 years to publish. 2. He used the time to test his hypothesis that life forms arose by descent from a common ancestor and that natural selection is a mechanism by which species can change and new species arise. 3. Darwin was forced to publish Origin of Species after reading a similar hypothesis by Alfred Russel Wallace. The Evidence of Evolution A. Common Descent 1. The hypothesis of common descent is supported by many lines of evidence. 2. The more varied the evidence, the more certain it becomes. 3. Darwin synthesized much of the current data but biochemical research was yet to come. B. Fossils Evidence 1. The fossil record is the history of life recorded by remains from the past. 2. Fossils are at least 10,000 years old and include skeletons, shells, seeds, insects trapped in amber, and imprints of leaves. 3. The fossil record traces history of life and allows us to study history of particular organisms. 4. Fossil evidence supports the common descent hypothesis; fossils can be linked over time because they reveal a similarity in form, despite observed changes. 5. Transitional forms reveal links between groups. a. Archeopteryx is an intermediate between reptiles and birds. b. Eustheopteron is an amphibious fish. c. Seymouria is a reptile-like amphibian. d. Therapsids were mammal-like reptiles. 6. The fossil record allows us to trace the history of the modern-day horse Equus. a. Earliest fossils show an ancestral Hyracotherium the size of a dog, with cusped lowcrowned molars, four toes on each front foot, three on each hind foot—all adaptations for forest living. b. When forests were replaced by grasslands, the intermediates were selected for durable grinding teeth, speed, etc. with an increase in size and decrease in toes. c. Living organisms resemble most recent fossils in the line of descent; underlying similarities allow us to trace a line of descent over time. C. Biogeographical Evidence 1. Biogeography studies the distribution of plants and animals worldwide. 2. Distribution of organisms is explained by related forms evolving in one locale and spreading to other accessible areas. a. Darwin observed South America had no rabbits; he concluded rabbits originated elsewhere. b. Biogeography explains the abundance of finch species on the Galápagos Islands lacking on the mainland. 3. Physical factors, such as the location of continents, determine where a population can spread. a. Cacti are restricted to North American deserts and euphorbia grow in African deserts. b. Marsupials arose when South America, Antarctica, and Australia were joined; Australia separated before placental mammals arose, so only marsupials diversified in Australia. D. Anatomical Evidence 1. Organisms have anatomical similarities when they are closely related because of common descent. a. Homologous structures in different organisms are inherited from a common ancestor. b. Analogous structures are inherited from unique ancestors and have come to resemble each other because they serve a similar function. c. Vertebrate forelimbs contain the same sets of bones organized in similar ways, despite their dissimilar functions. 2. Vestigial structures are remains of a structure that was functional in some ancestors but is no longer functional in the organism in question. a. Most birds have well-developed wings; some bird species have reduced wings and do not fly. b. Humans have a tailbone but no tail. c. Presence of vestigial structures is explained by the common descent hypothesis; these are traces of an organism's evolutionary history. 3. Embryological development reveals a unity of plan. a. During development, all vertebrates have a post-anal tail and paired pharyngeal pouches. i. In fishes and amphibian larvae, the pouches become gills. ii. In humans, first pair of pouches becomes a cavity of middle ear and auditory tube; second pair becomes tonsils, while third and fourth pairs become thymus and parathyroid glands. iii. The above features are explained if fishes are ancestral to other vertebrate groups. E. Biochemical Evidence 1. Almost all living organisms use the same basic biochemical molecules, e.g., DNA, ATP, and many identical or nearly identical enzymes. 2. Organisms utilize the same DNA triplet code and the same 20 amino acids in their proteins. 3. Many organisms share the same introns and types of repeats, which is remarkable since there is no obvious functional reason why these components need to be so similar. 4. This is substantiated by the analysis of the degree of similarity in amino acids for cytochrome c among organisms. 5. These similarities can be explained by descent from a common ancestor. 6. Life's vast diversity has come about by only a slight difference in the same genes. F. Because it is supported by so many lines of evidence, evolution is no longer considered a hypothesis. 1. Evolution is one of the great unifying theories of biology, similar in status to the germ theory of disease in medicine. 2. In science, theory is reserved for those conceptual schemes that are supported by a large number of observations or a large amount of experimental evidence and have not been found lacking. The modern synthesis of evolutionary ideas resulted when genetics and Darwin's theory were combined. The realization that DNA produced proteins and their proteins were acted upon by the environment is the central idea in modern evolutionary theory. Understanding the Hardy Weinberg law is important understanding how population genetics works and helps demonstrate the mechanisms required for evolutionary change the population. 23 – Microevolution - the evolution of populations 1. It was not until the 1930s that population geneticists were able to apply the principles of genetics to populations and thus to recognize when evolution had occurred. 2. A population is all of the members of a single species occupying a certain area at the same time. 3. Evolution that occurs within a population is called microevolution. 4. Population genetics studies the variation in alleles in a gene pool. 5. The gene pool is the total of all the alleles in a population; it is described in terms of gene frequencies. 6. Neither dominance nor sexual reproduction changes allele frequencies. 7. The Hardy-Weinberg principle a. This principle states an equilibrium of allele frequencies in a gene pool (using a formula p2 + 2pq + q2) remains in effect in each succeeding generation of a sexually reproducing population if five conditions are met. i. No mutation: no allelic changes occur, or changes in one direction are balanced by changes in the other direction. ii. No gene flow: migration of alleles into or out of the population does not occur. iii. Random mating: individuals pair by chance and not according to their genotypes or phenotypes. iv. No genetic drift: the population is large so changes in allele frequencies due to chance are insignificant. v. No selection: no selective force favors one genotype over another. b. In real life, conditions of the Hardy-Weinberg law are rarely if ever met, and allele frequencies in the gene pool of a population do change from one generation to the next, resulting in evolution. c. Any change of allele frequencies in a gene pool of a population signifies that evolution has occurred. d. The Hardy-Weinberg law tells us what factors cause evolution—those that violate the conditions listed. e. A Hardy-Weinberg equilibrium provides a baseline by which to judge whether evolution has occurred. B. C. D. E. f. Hardy-Weinberg equilibrium is a constancy of gene pool frequencies that remains across generations. 8. Industrial Melanism a. The case of the peppered moths provides a case study in a shift in phenotype frequencies under selection. b. Before trees became coated with soot from air pollution, the percentage of dark-colored moths was 10%. c. With birds acting as a selective agent, the light colored moths were reduced while darkcolored moths were better adapted to survive on the darkened trees. d. The last generation observed has 80% dark-colored moths. Causes of Microevolution 1. Genetic Mutations a. Many traits in organisms are polymorphic, i.e., two or more distinct phenotypes are present in the population due to mutated genes. b. Analysis of Drosophila enzymes indicates they have multiple alleles at least at 30% of their gene loci. c. In humans, freckles are an example of polymorphism, as are the ABO blood types. d. Mutations may not immediately affect the phenotype. e. Mutations can be beneficial, neutral, or harmful; a seemingly harmful mutation that requires Daphnia to live at higher temperatures becomes advantageous when the environment changes. f. Specific recombinations of alleles may be more adaptive than other combinations. Gene Flow 1. Gene flow (gene migration) is the movement of alleles among populations by migration of breeding individuals. 2. Gene flow can increase variation within a population by introducing novel alleles produced by mutation in another population. 3. Continued gene flow decreases diversity among populations, causing gene pools to become similar. 4. Gene flow among populations can prevent speciation from occurring. Nonrandom Mating 1. Random mating involves individuals pairing by chance, not according to genotype or phenotype. 2. Nonrandom mating involves inbreeding and assortative mating. 3. Inbreeding is mating between relatives to a greater extent than by chance. a. Inbreeding does not change the allele frequencies. b. However, inbreeding decreases the proportion of heterozygotes. c. Inbreeding increases the proportions of both homozygotes at all gene loci. d. In human populations, inbreeding increases the frequency of recessive abnormalities. 4. Assortative mating occurs when individuals mate with those that have the same phenotype. a. Assortative mating divides a population into two phenotypic classes with reduced gene exchange. b. Homozygotes for gene loci that control a trait increase, and heterozygotes for these loci decrease. 5. Sexual selection occurs when males compete for the right to reproduce and the female selects males of a particular phenotype. Genetic Drift 1. Genetic drift refers to changes in allele frequencies of a gene pool due to chance. 2. Genetic drift occurs in both large and small populations; large populations suffer less sampling error. 3. Genetic drift causes isolated gene pools to become dissimilar; some alleles are lost and others are fixed or are the only allele in the population. 4. Genetic drift occurs when founders start a new population, or after a genetic bottleneck with interbreeding. a. The bottleneck effect prevents most genotypes from participating in production of the next generation. i. The bottleneck effect is caused by a severe reduction in population size due to a natural disaster, predation, or habitat reduction. ii. The bottleneck effect causes a severe reduction in the total genetic diversity of the original gene pool. iii. The cheetah bottleneck causes relative infertility because alleles were lost due to intense inbreeding when populations were reduced in earlier times. b. The founder effect is an example of genetic drift where rare alleles or combinations occur in higher frequency in a population isolated from the general population. i. This is due to founding individuals containing a fraction of total genetic diversity of the original population. ii. Which particular alleles are carried by the founders is dictated by chance alone. iii. As an example, dwarfism is much higher in a Pennsylvania Amish community due to a few German founders. Natural Selection o Natural selection is the process that results in adaptation of a population to the environment. 1. Natural selection requires a. variation (i.e., the members of a population differ from one another), b. inheritance (i.e., many of the differences between individuals in a population are heritable genetic differences), c. differential adaptedness (i.e., some differences affect how well an organism is adapted to its environment), and d. differential reproduction (i.e., better adapted individuals are more likely to reproduce). 2. Fitness is the extent to which an individual contributes fertile offspring to the next generation. 3. Relative fitness compares the fitness of one phenotype to another. B. Types of Selection. 1. Directional selection occurs when an extreme phenotype is favored; the distribution curve shifts that direction. a. A shift to dark-colored peppered moths from light-colored correlated with increasing pollution. b. Drug-resistant strains of bacteria are a serious health threat and represent this type of selection. c. Increases in insecticide-resistant mosquitoes and resistance of the malaria protozoan Plasmodium to medications are also examples of directional selection. d. The gradual increase in the size of the modern horse, Equus, correlates with a change in the environment from forest-like conditions to grassland conditions. 2. Stabilizing selection occurs when extreme phenotypes are eliminated and the intermediate phenotype is favored. . The average number of eggs laid by Swiss starlings is four or five. a. If the female lays more or less than this number, fewer survive. b. Genes determining the physiology of yolk production and behavior are involved in clutch size. 3. Disruptive selection occurs when extreme phenotypes are favored and can lead to more than one distinct form. . British snails (Cepaea nemoralis) vary because a wide range causes natural selection to vary. a. In forest areas, thrushes feed on snails with light bands. b. In low-vegetation areas, thrushes feed on snails with dark shells that lack light bands. C. Maintenance of Variations 0. Populations always show some genotypic variation; populations that lack variation may not be able to adapt to new conditions. 1. How is variation maintained in the face of constant selection pressure? 2. The following forces promote genetic variation. . Mutation creates new alleles and genetic recombination still combines these alleles. a. Gene flow among small populations introduces new alleles. b. Natural selection, such as disruptive selection, itself sometimes promotes variation. 3. Diploidy and the Heterozygote . Only alleles that cause phenotypic differences are subject to natural selection. a. In diploid organisms, a heterozygote shelters rare recessive alleles that would otherwise be selected out. b. Even when selection reduces the recessive allele frequency from 0.9 to 0.1, the frequency in the heterozygote remains the same and remains a resource for natural selection in a new environment. 4. Sickle-Cell Disease . In sickle-cell disease, heterozygotes are more fit in malaria areas because the sickle-cell trait does not express unless the oxygen content of the environment is low; but the malaria agent causes red blood cells to die when it infects them (loss of potassium). a. Some homozygous dominants are maintained in the population but they die at an early age from sickle-cell disease. b. Some homozygotes are maintained in the population for normal red blood cells, but they are vulnerable to malaria. Macroevolution Macroevolution refers to any evolutionary change at or above the species level. Speciation is the splitting of one species into two or more species or the transformation of one species into a new species over time; speciation is the final result of changes in gene pool allele and genotypic frequencies. A. What is a Species? 1. Linnaeus separated species based on morphology, i.e., their traits differed; Darwin saw that similar species are related by common descent. 2. Ernst Mayr (1942) developed the biological species concept: a species is a group of actually or potentially interbreeding populations that are reproductively isolated from other such groups. 3. The biological definition of a species says that the members of one species interbreed and have a shared gene pool, and each species is reproductively isolated from every other species. 4. Gene flow occurs between populations of one species but not between populations of different species. 5. Biochemical genetics uses DNA hybridization techniques to determine relatedness of organisms; the phylogenetic species concept uses DNA/DNA comparisons. B. Reproductive Isolating Mechanisms 1. For two species to be separate, gene flow must not occur between them. 2. A reproductive isolating mechanism is any structural, functional, or behavioral characteristic that prevents successful reproduction from occurring. 3. Prezygotic ("before formation of a zygote") isolating mechanisms are anatomical or behavioral differences between the members of two species that prevent mating or make it unlikely fertilization will take place if mating occurs. a. Habitat isolation occurs when two species occupy different habitats, even within the same geographic range, so that they are less likely to meet and to attempt to reproduce. b. Temporal isolation occurs when two species live in the same location, but each reproduces at a different time of year, and so they do not attempt to mate. c. Behavioral isolation results from differences in mating behavior between two species. d. Mechanical isolation is the result of differences between two species in reproductive structures or other body parts, so that mating is prevented. e. Gamete isolation includes incompatibility of gametes of two different species so they cannot fuse to form a zygote; an egg may have receptors only for the sperm of its own species or a plant stigma prevents completion of pollination. 4. Postzygotic ("after formation of a zygote") isolating mechanisms prevent development of a hybrid after mating has taken place. a. Zygote mortality is when hybrids (offspring of parents of two different species) do not live to reproduce. b. Hybrid sterility occurs when the hybrid offspring are sterile (e.g., mules). c. In F2 fitness, the offspring are fertile but the F2 generation is sterile. C. Modes of Speciation 1. Allopatric speciation occurs when new species result from populations being separated by a geographical barrier that prevents their members from reproducing with each other. a. First proposed by Ernst Mayr of Harvard University. b. While geographically isolated, variations accumulate until the populations are reproductively isolated. c. First postzygotic isolation occurs, then prezygotic reproductive isolation occurs. 2. Sympatric speciation would occur when members of a single population develop a genetic difference (e.g., chromosome number) that prevents them from reproducing with the parent type. a. The main example of sympatric speciation is in plants. b. Failure to reduce chromosome number produces polyploid plants that reproduce successfully only with polyploids. c. Backcrosses with diploids are sterile. D. Adaptive Radiation 1. Adaptive radiation is a rapid development from a single ancestral species of many new species. 2. The case of Darwin's finches illustrates the adaptive radiation of 13 species from one founder mainland finch. 3. On the Hawaiian Islands, a wide variety of honeycreepers descended from one goldfinchlike ancestor; Hawaii is also the home of the silversword plants that radiated from ancestral tarweeds. 25 - Origin of Life o Chemical evolution is the increase in complexity of chemicals that led to the first cells. 1. Today, we say that "life only comes from life." 2. However, the first cells had to arise from an increased complexity of chemicals. B. The Early Earth 1. The Earth came into being about 4.6 BYA (BYA). 2. Heat from gravitation and radioactivity formed the Earth in several layers with iron and nickel in a liquid core, silicate minerals in a semi-liquid mantle, and upwellings of volcanic lava forming the first crust. 3. The Earth's mass provides a gravitational field strong enough to hold an atmosphere. 4. Early Earth's atmosphere differed from the current atmosphere, consisting of: a. water vapor, b. nitrogen, c. carbon dioxide, d. small amounts of hydrogen, methane, ammonia, hydrogen sulfide, and carbon monoxide. 5. The early atmosphere was formed by volcanic out-gassing characteristic of the young Earth. 6. The early atmosphere contained little free oxygen (O2) and was probably a reducing atmosphere with little free oxygen; a reducing atmosphere lacks free O2 and allows formation of complex organic molecules. 7. The early Earth was so hot that H2O only existed as a vapor in dense, thick clouds. 8. As the Earth cooled, H2O vapor condensed to form liquid H2O, and rain collected in oceans. 9. The Earth's distance from the sun allows H2O to exist in all phases: solid, liquid, and gas. 10. NASA photos seem to confirm that Earth is bombarded by comets adding substantial water vapor. C. Monomers Evolve 0. Comets and meteorites, perhaps carrying organic chemicals, have pelted the Earth throughout history. 1. A meteorite from Mars (ALH84001) that landed on Earth 13,000 years ago, may have fossilized bacteria. 2. Oparin/Haldane Hypothesis (1920s) . Oparin/Haldane independently suggested organic molecules could be formed in the presence of outside energy sources using atmospheric gases. a. Experiments performed by Miller and Urey (1953) showed experimentally that these gases (methane, ammonia, hydrogen, water) reacted with one another to produce small organic molecules (amino acids, organic acids). 3. Lack of oxidation and decay allowed organic molecules to form a thick, warm organic soup. 4. Ammonia may have been scarce in the early atmosphere; undersea thermal vents, which line ocean ridges, might have been responsible for converting nitrogen to ammonia. D. Polymers Evolve 0. Newly formed organic molecules polymerized to produce larger molecules. . Wachtershauser and Huber formed peptides using iron-nickel sulfides under ventlike conditions. a. Such minerals have a charged surface that attracts amino acids and provides electrons so they bond together. 1. Protein-first Hypothesis . Sidney Fox demonstrated amino acids polymerize abiotically if exposed to dry heat. a. Amino acids collected in shallow puddles along the rocky shore; heat of the sun caused them to form proteinoids (i.e., small polypeptides that have some catalytic properties). b. When proteinoids are returned to water, they form cell-like microspheres composed of protein. c. This assumes DNA genes came after protein enzymes; DNA replication needs protein enzymes. 2. The Clay Hypothesis . Graham Cairns-Smith suggests that amino acids polymerize in clay, with radioactivity providing energy. a. Clay attracts small organic molecules and contains iron and zinc atoms serving as inorganic catalysts for polypeptide formation. b. Clay collects energy from radioactive decay and discharges it if temperature or humidity changes. c. If RNA nucleotides and amino acids became associated so polypeptides were ordered by and helped synthesize RNA, then polypeptides and RNA arose at the same time. 3. RNA-first Hypothesis . Only the macromolecule RNA was needed at the beginning to lead to the first cell. a. Thomas Cech and Sidney Altman discovered that RNA can be both a substrate and an enzyme. b. RNA would carry out processes of life associated with DNA (in genes) and protein enzymes. c. Supporters of this hypothesis label this an "RNA world" 4 BYA. E. A Protocell Evolves 0. Before the first true cell arose, there would have been a protocell or protobiont. 1. A protocell would have a lipid-protein membrane and carry on energy metabolism. 2. Fox showed that if lipids are made available to microspheres, lipids become associated with microspheres producing a lipid-protein membrane. 3. Oparin demonstrated a protocell could have developed from coacervate droplets. . Coacervate droplets are complex spherical units that spontaneously form when concentrated mixtures of macromolecules are held in the right temperature, ionic composition, and pH. a. Coacervate droplets absorb and incorporate various substances from the surrounding solution. b. In a liquid environment, phospholipid molecules spontaneously form liposomes, spheres surrounded by a layer of phospholipids; this is called the "membrane-first" hypothesis. c. A protocell could have contained only RNA to function as both genetic material and enzymes. 4. If a protocell was a heterotrophic fermenter living on the organic molecules in the organic soup that was its environment, this would indicate heterotrophs preceded autotrophs. . A heterotroph is an organism that cannot synthesize organic compounds from inorganic substances and therefore must take in preformed organic compounds. a. An autotroph is an organism that makes organic molecules from inorganic nutrients. 5. If the protocell evolved at hydrothermal vents, it would be chemosynthetic and autotrophs would have preceded heterotrophs. 6. The first protocells may have used preformed ATP, but as supplies dwindled, natural selection would favor cells that could extract energy from carbohydrates to transform ADP to ATP. 7. Since glycolysis is a common metabolic pathway in living things, it evolved early in the history of life. 8. As there was no free O2, it is assumed that protocells carried on a form of fermentation. 9. The first protocells had a limited ability to break down organic molecules; it took millions of years for glycolysis to evolve completely. 10. Fox has shown that a microsphere has some catalytic ability; Oparin found that coacervates incorporate enzymes if they are available in the medium. F. A Self-Replication System Evolves 0. In living systems, information flows from DNA → RNA → protein; it is possible that this sequence developed in stages. 1. The RNA-first hypothesis suggests that the first genes and enzymes were RNA molecules. . These genes would have directed and carried out protein synthesis. a. Ribozymes are RNA that acts as enzymes. b. Some viruses contain RNA genes with a protein enzyme called reverse transcriptase that uses RNA as a template to form DNA; this could have given rise to the first DNA. 2. The protein-first hypothesis contends that proteins or at least polypeptides were the first to arise. . Only after the protocell develops complex enzymes could it form nucleic acids from small molecules. a. Because a nucleic acid is complicated, the chance that it arose on its own is minimal. b. Therefore, enzymes are needed to guide the synthesis of nucleotides and then nucleic acids. 3. Cairns-Smith suggests that polypeptides and RNA evolved simultaneously. . The first true cell would contain RNA genes that replicated because of the presence of proteins; they become associated in clay in such a way that the polypeptides catalyzed RNA formation. a. This eliminates the chicken-and-egg paradox; both events happen at the same time. 4. Once the protocell was capable of reproduction, it became a true cell and biological evolution began. . After DNA formed, the genetic code still had to evolve to store information. a. Because the current code is subject to fewer errors than other possible codes, and because it minimizes mutations, it likely underwent a natural selection process. 5. Most biologists suspect life evolved in basic steps. . Abiotic synthesis of organic molecules such as amino acids occurred in the atmosphere or at hydrothermal vents. a. Monomers joined together to form polymers at seaside rocks or clay, or at vents; the first polymers could have been proteins or RNA or both. b. Polymers aggregated inside a plasma membrane to make a protocell that had limited ability to grow; if it developed in the ocean it was a heterotroph, if at a hydrothermal vent, a chemoautotroph. c. Once the protocell contained DNA genes or RNA molecules, it was a true cell. 25 - History of Life A. Fossils Tell a Story 1. A fossil is the remains or traces of past life, usually preserved in sedimentary rock. 2. Most dead organisms are consumed by scavengers or decompose. 3. Paleontology is the study of fossils and the history of life, ancient climates, and environments. 4. Sedimentation has been going on since the Earth was formed; it is an accumulation of particles forming a stratum, a recognizable layer in a stratigraphic sequence laid down on land or in water. 5. The sequence indicates the age of fossils; a stratum is older than the one above it and younger than the one below it. B. Relative Dating of Fossils 1. Strata of the same age in England and Russia may have different sediments. 2. However, geologists discovered that strata of the same age contain the same fossils, termed index fossils. 3. Therefore, fossils can be used for the relative dating of strata. 4. A particular species of fossil ammonite is found over a wide range and for a limited time period; therefore, all strata in the world that contain this ammonite are of the same age. 5. However, relative dating does not establish the absolute age of fossils in years. C. Absolute Dating of Fossils 1. Absolute dating relies on radioactive dating to determine the actual age of fossils. 2. Radioactive isotopes have a half-life, the time it takes for half of a radioactive isotope to change into a stable element. 3. Carbon 14 (14C) is a radioactive isotope contained within organic matter. a. Half of the carbon 14 (14C) will change to nitrogen 14 (14N) every 5,730 years. b. Comparing 14C radioactivity of a fossil to modern organic matter calculates the age of the fossil. c. After 50,000 years, the 14C radioactivity is so low it cannot be used to measure age accurately. 4. It is possible to determine the ratio of potassium 40 (40K) and argon 40 to date rocks and infer the age of a fossil. D. The Precambrian Geologists have devised the geological timescale, which divides the history of Earth into eras, and then periods and epochs. 1. Life arose in the Precambrian Era. a. The Precambrian encompasses 87% of the geologic time scale. b. Early bacteria probably resembled the archaea that live in hot springs today. c. 3.8 BYA, the first chemical fingerprints of complex cells occur; at 3.46 BYA, photosynthetic prokaryotic cells appear. d. Boulders called stromatolites from this early time resemble living stromatolites with cyanobacteria in the outer surface. e. Oxygen-releasing photosynthesis by cyanobacteria in stromatolites caused the atmosphere to become oxidizing rather than reducing. f. By 2 BYA, oxygen levels were high enough that anaerobic prokaryotes were declining. g. Accumulation of O2 caused extinction of anaerobic organisms and the rise of aerobic organisms. h. O2 forms ozone or O3 in the upper atmosphere, contributing to the ozone shield and blocking ultraviolet radiation from reaching the Earth's surface; this allowed organisms to live on land. 2. Eukaryotic Cells Arise a. The eukaryotic cell, which arose 2.1 BYA, is always aerobic and contains a nucleus and organelles. b. The Endosymbiotic Hypothesis i. Mitochondria were probably once free-living aerobic prokaryotes. ii. Chloroplasts were probably once free-living photosynthetic prokaryotes. iii. A nucleated cell probably engulfed these prokaryotes that became various organelles. iv. Cilia and flagella may have originated from slender undulating prokaryotes that attached to the host cell. 3. Multicellularity Arises a. It is not known exactly when multicellular organisms appeared; they would have been microscopic. b. Separating germ cells from somatic cells may have contributed to the diversity of organisms. c. Fossils of the Ediacara Hills of South Australia, from about 600-545 MYA, were softbodied early invertebrates. i. These bizarre animals lived on mudflats in shallow marine waters. ii. They lacked internal organs and could have absorbed nutrients from the sea. E. The Paleozoic Era 1. The Paleozoic Era lasted over 300 million years and was a very active period with three major mass extinctions. a. An extinction is the total disappearance of a species or higher taxonomic group. b. Mass extinction is the disappearance of a large numbers of species or higher groups in a short geological time, just a few million years. 2. Cambrian Animals a. The Cambrian Period saw invertebrates flourish; invertebrates lack a vertebral column. b. Today's invertebrates all trace their ancestry to the Cambrian Period, and possibly earlier. c. A molecular clock, based on a fixed rate of changes in base pair sequences, allows us to trace backward how long current species have evolved separately. d. Why fossils are easy to find in the Cambrian but not before is a complex question; most likely the animals evolved earlier but without outer skeletons. e. Cambrian seafloors were dominated by trilobites, now extinct, that had armored exoskeletons. f. Perhaps the evolution of exoskeletons was due to the presence of plentiful O2 in the atmosphere. g. A skeleton may have been due to the increased pressures of predation. 3. Invasion of Land a. Early in the Ordovician Period, marine algae expanded to freshwater. b. In the Silurian Period, vascular plants invaded land and later flourished in warm swamps in the Carboniferous Period. c. Spiders, centipedes, mites and millipedes all preceded the appearance of insects on land. d. The appearance of wings on insects in the Carboniferous Period allowed insects to radiate into a diverse group. e. The vertebrate line of descent began in the early Ordovician Period. f. The Devonian Period is called the Age of Fishes and saw jawless and then jawed fishes, including both cartilaginous and ray-finned fishes. g. The Carboniferous Period was an age of coal-forming forests with an abundance of club mosses, horsetails, and ferns. i. It is called the "Age of the Amphibians" because amphibians diversified at this time. ii. Early vascular plants and amphibians were larger and more abundant during the Carboniferous Period; a climate change to colder and drier began the process that produced coal. F. The Mesozoic Era 1. Although there was a mass extinction at the end of the Paleozoic, evolution of some plants and animals continued into the Triassic, the first period of the Mesozoic Era. 2. The Triassic period a. Gymnosperms flourished, especially cycads; the Triassic and Jurassic are called the "Age of Cycads." b. One group of reptiles, the therapsids, had the first mammal features. c. Reptiles, originating in the Permian, underwent adaptive radiation. 3. The Jurassic Period a. Many dinosaurs flourished in the sea, on land and in air. b. Controversy surrounds dinosaurs being ectothermic or endothermic. 4. The Cretaceous Period a. A new Chinese fossil, Jeholodens, reveals an early mammal with a long snout but sprawling reptile-like hind limbs. b. The era of dinosaurs ended in a mass extinction in which dinosaurs, most reptiles, and many marine organisms perished. G. The Cenozoic Era 1. The Cenozoic Era is divided into the Tertiary and the Quaternary Periods. 2. During the Cenozoic Era, mammals with hair and mammary glands diversified and human evolution began. 3. Mammalian Diversification a. During the Paleocene Epoch, mammals were small and resembled rats. b. In the Eocene Epoch, all of the modern orders of mammals had developed. c. Many of the types of herbivores and carnivores of the Oligocene Epoch are extinct today. 4. Evolution of Primates a. Flowering plants were diverse and plentiful by the Cenozoic Era; primates were adapted to living in flowering trees. b. The first primates were small squirrel-like animals; from them evolved the first monkeys and apes. c. Apes diversified during the Miocene and Pliocene Epochs; this includes the first hominids, the group that includes humans. d. During the Tertiary Period, the world's climate cooled with the last two epochs known as the Ice Age. e. The Pleistocene Epoch saw many large sloths, beavers, wolves, bison, woolly rhinoceroses, mastodons, and mammoths; modern humans arose and may have contributed to extinction. Factors That Influence Evolution A. Continental Drift 1. Earth's crust is dynamic, not immobile as was once thought. 2. In 1920, German meteorologist Alfred Wegener presented data from across disciplines supporting continental drift. 3. Continental drift was confirmed in the 1960s; the continents moved with respect to one another. 4. During the Permean Period, the continents were joined to form one supercontinent called Pangaea which later divided into Gondwana and Laurasia and then split to form today's configuration. 5. Continental drift explains why the coastlines of several continents (e.g., the outline of the west coast of Africa and that of the east coast of South America) are mirror images of each other. 6. The same geological structures (e.g., mountain ranges) are found in many areas where continents once touched. 7. Continental drift explains unique distribution patterns of several fossils (e.g., species of the seed fern Glossopteris). 8. Continental drift also explains why some fossils (e.g., reptiles Cynognathus and Lystrosaurus) are found on different continents. 9. Continental drift explains why Australia, South America, and Africa have distinctive mammals; current mammalian biological diversity is the result of isolated evolution on separate continents. B. Plate Tectonics 1. Plate tectonics is the study of the behavior of the Earth's crust in terms of moving plates that are formed at ocean ridges and destroyed at subduction zones. 2. Ocean ridges are ridges on ocean floors where oceanic crust forms; regions in oceanic crust where molten rock rises and material is added to the ocean floor result in seafloor spreading. 3. Seafloor spreading is the lateral movement of oceanic crust away from ocean ridges due to material added to the ocean floor. 4. Subduction zones are regions where oceanic crust collides with the continental crust, causing the oceanic crust to descend into the mantle where it is melted. 5. Where the ocean floor is at the leading edge of a plate, a deep trench forms bordered by volcanoes or volcanic island chains. 6. Two continents colliding form a mountain range (e.g., the Himalayas are the result of the collision of India and Eurasia). 7. Transform boundaries are regions where two crustal plates meet and scrape past one another resulting in relatively frequent earthquakes. C. Mass Extinctions 1. Five mass extinctions occurred at the ends of the Ordovician, Devonian, Permian, Triassic, and Cretaceous periods. 2. Mass extinctions have been attributed to tectonic, oceanic, and climatic changes. 3. Walter and Louis Alvarez proposed that the Cretaceous extinction was due to a bolide (an asteroid that explodes producing meteorites) striking the Earth. a. A layer of iridium soot has been identified in the Cretaceous clay, the correct strata. b. A huge crater near the Yucatan is the impact site. c. The effect would have resembled a worldwide atomic explosion. 4. David Raup and John Sepkoski proposed that marine fossils show mass extinctions every 26 million years, in periodicity with astronomical movement through the galaxy. 5. Continental drift contributed to Ordovician extinction; Gondwanaland arrived at the south pole and glaciers chilled oceans and land until Gondwanaland drifted away from the pole. 6. The Devonian extinction may have been a bolide event; this saw an end to 70% of the marine invertebrates; other possibilities include drifting back toward the south pole. 7. The Permian extinction was very severe; 90% of ocean species and 70% of land species disappeared perhaps due to an excess of carbon dioxide due to a change in ocean circulation due to a lack of polar ice caps. 8. The Triassic extinction has been attributed to meteorite collision with Earth; a crater in Central Quebec may have been the impact site.