File
advertisement

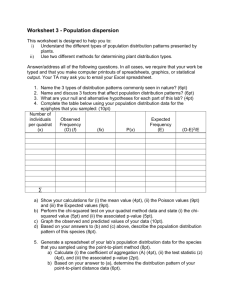
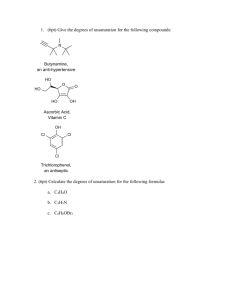
1. (3pt) Draw a s orbital, a p orbital, an a single sp3 orbital in the spaces provided (assume the nucleus is at the center of the axis) 2. (4pt) Fill the valence electron shells for the following atoms: 2p Nitrogen, N 2s 2p Carbon, C 2s 3. (3pt) Write the ground state electron configurations for the following. Boron, B Sodium, Na Oxide ion, O2- 4. (3pt) Draw Lewis structures for the following molecules using dots to indicate both covalent bonds and lone pairs. AlCl3 H3CCN N3- 5. (3pt) Ethylene (CH2CH2) has a single C-C double bond. Draw the p orbitals on ethylene and indicate how they interact to form a bond (only bonds are shown below) H H C C H H 6. (3pt) Explain why cyclopropane is a relatively unstable molecule. H H C H C H H C H 7. (6pt) Give the hybridization for the circled non-hydrogen atoms Norethynodrel The first oral contraceptive 3 Vitamin B6 CH 6 4 OH H O C H CH2 H O C H HO HO O 2 N 5 1 1______________________ 2______________________ 3______________________ 4______________________ 5______________________ 6______________________ 8. (3pt) Arrange the following molecules in increasing bond strength. HC N H3C NH2 H2 C Circle the molecule that has the shortest C-N bond. NH OH P O 9. (3pt) Give the bond angles for each of the indicated carbons: Norethynodrel 3 OH 2 H O H C 1. ________________________ CH 2. ________________________ C 3. ________________________ H 1 10. (6pt) Indicate the direction of each molecule’s overall dipole moment. Leave molecules without a dipole moment blank. H Br C H C H O Cl Cl Al Cl S C OH Li O H3C H3C H3C CH3 CH3 CH3 CH3 11. (4pt) Dichloromethane (CH2Cl2) has a dipole moment, but carbon tetrachloride (CCl4) does not, explain why. 12. (4pt) Calculate the formal charges for the following (lone pairs are shown, charges are not): O * O N F O H N_____________ *O____________ F B O F B____________ O_____________ 13. (7pt) Show all resonance forms for the following using correct arrows indicate electron movement and differentiate between resonance forms: O O O O H H H 14.(3pt) Draw an arrow (doesn’t matter what kind) in the direction you would expect each equilibrium to proceed (pKa’s are given for each acidic species). + + N H N 15 44 O H3 O+ O + + H2O -1.7 O OH 4.76 O + + OH O O N HN 17 14.7 O O 15. (2pt) Circle which has the strongest conjugate base. NO2 or NH2 O or O2N NO2 pKa 8.3 pKa 21 NH3 pKa 41 pKa -9.3 16. (6pt) Use correct arrows to show the electron flow and predict the products of these two reactions + OH N H H H N Cl Cl Al + H Cl 17. (4pt) Indicate which molecules are isomers by circling the pair. Leave non-isomers pairs blank. O and OH and O O and H O HO O HO O and O 18. (5pt) List all intermolecular forces you would expect in the following compounds, circle the compounds you would expect to be hydrophilic. O OH Cl C Cl Cl Cl O 19. (6pt) Name the indicated functional groups Prozac 1 Vitamin B6 3 H N 6 5 O H O HO 4 F HO O 2 F F N 1______________________ 2_____________________ 3______________________ 4______________________ 5______________________ 6______________________ OH P O 20. (6pt) Draw the following molecules: sec-butyl chloride (an alkyl halide) iso-pentyl alcohol tert-butyl thiol 21. (9pt) Name the following molecules: 22. (7pt) Consider 1-bromoethane (BrCH2CH2CH3) Draw a Newman projection through the C2-C3 bond in the following conformations: Anti Gauche Two different eclipsed Given the following information, calculate the barrier to rotation of the most strained eclipsed conformer. Interaction H-H eclipsed C-H eclipsed C-C eclipsed Br-C eclipsed Energy 4.0 kJ/mol 6.0 kJ/mol 11.0 kJ/mol 13.0 kJ/mol Bonus (4pt) Give a molecule of the day’s chemical name and describe its use. Bonus (2pt) Below is a molecule used on a promotional bag for the 2007 ACS Regional Meeting. Circle and identify the mistake in this molecule: OH O S